3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2018; 9(11):1973-1977. doi:10.7150/jca.24782 This issue Cite
Research Paper
Aerosol Immunotherapy with or without Cisplatin for metastatic lung cancer non-small cell lung cancer disease: In vivo Study. A more efficient combination
1. 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece
2. Pulmonary Department-Oncology Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece
3. Department of Respiratory and Critical Care Medicine, Changhai Hospital, Second Military Medical University, Shanghai, China
4. Department of Respiratory Diseases, The Affiliated Jiangning hospital of Nanjing Medical University, Nanjing, China
5. Department of Pharmacology & Clinical Pharmacology, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
6. Anesthesiology Department, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece
7. Ear, Nose and Throat Department, "Saint Luke" Private Hospital, Panorama, Thessaloniki, Greece
8. Medical Clinic I, "Fuerth" Hospital, University of Erlangen, Fuerth, Germany
9. Thoracic Surgery Department, University General Hospital of Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece
# contributed equally to this work.
Received 2018-1-7; Accepted 2018-3-7; Published 2018-4-30
Abstract
Lung cancer is the leading cause of cancer death after prostate cancer for males and breast cancer for females. There are novel therapies in the past five years such as; tyrosine kinase inhibitors and most recently in the last two years immunotherapy. Immunotherapy is currently being investigated if it can be administered alone or in combination. Previously we have investigated whether immunotherapy compounds can be produced as aerosols, and in the current study we investigated the safety and efficiency independently of the programmed death-ligand 1. The aerosol administration of both cisplatin and nivolumab is possible. The combination of the two drugs has a synergistic effect and therefore should be considered an option. Time of administration for immunotherapy is also very important.
Keywords: lung cancer, aerosol, immunotherapy, NSCLC, nivolumab, ipilimumab, pembrolizumab, programmed death-ligand 1
Introduction
Lung cancer is diagnosed at a late stage due to the lack of symptoms. Although we have novel diagnostic tools such as the radial-ebus and convex-probe-ebus there are no guidelines or screening methods that are globally accepted for early lung cancer screening.[1-7] In the past five years based on the pharmacogenomics of the tumor we have novel therapies such as; tyrosine kinase inhibitors (TKIs) and immunotherapy for lung cancer.[8] Regarding small cell lung cancer we have treatment limitation for now with chemotherapy and radiotherapy.[9-13] Targeted therapy based on the expression of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and recently for proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B (BRAF) and proto-oncogene tyrosine-protein kinase ROS (ROS-1) is available.[14] In the past year immunotherapy with nivolumab and pembrolizumab have been approved as lung cancer treatment for metastatic lung cancer disease.[15] Ipilimumab is another drug that is currently being investigated for lung cancer treatment in combination with other immunotherapy drugs.[16] Aerosol treatment for systematic diseases has been previously established and is being used for diabetes, chronic obstructive pulmonary disease (COPD), cystic fibrosis and pulmonary hypertension.[17-21] Previously we have modified nivolumab, ipilimumab and pembrolizumab to be produced as aerosol. In this study we observed that nivolumab had the smallest aerosol droplet size 1.89μm MMAD. Moreover; in one of our previous publications we have designed and used a cage for BALBC mice in order to administer aerosol therapies.[22] In the current study we will evaluated the safety and efficacy of aerosol nivolumab in a lewis lung carcinoma cell line model.
Αnimals and Methods
Mice
One hundred BALBC mice age 7-8 weeks old were purchased for the experiment and were divided in five groups. The mice included were isolated (1 per cage) in a temperature-controlled room on 12-hour light-dark cycle and were allowed free access to food and water. The Lewis lung carcinoma cell line was obtained by ATCC (CRL-1642™). The cells were routinely cultured in 25-cm2 tissue culture flasks containing RPMI (ATCC, 30-2002) supplemented with 10% fetal bovine serum (Biochrom) according to the supplier's instruction. The cell line was incubated at 37° C in 5% CO2. The doubling time of the cell line was 21 hours.[23] At confluence, cells were harvested with 0.25% trypsin and then were re-suspended at 1,5×106 cells in 0.15 ml PBS (Phosphate Buffered Saline, Dulbecco, Biochrom) which was injected in the left back foot of the mice. The foot was inoculated subcutaneously (27-guage needle, 1,5×106 cells). The tumor volume was measured once weekly using bidimensional diameters (caliper) with the equation V=1/2ab2, where the a represents the length and b the width (mm3). The tumor was grown on the left back foot of the mice. The animals were randomly divided into five groups of 20, when the tumor volume reached ~100mm3. The mice were divided into five groups as follows: a) control group (no therapy), b) only aerosol nivolumab, c) only aerosol cisplatin, d) aerosol nivolumab and after three days aerosol cisplatin, e) aerosol cisplatin and after three days aerosol nivolumab.
Immunotherapy
Nivolumab 10mg/ml from Bristol-Myers-Squibb was purchased for the experiment with the dosage being administered at 3mg/kg, and calculated according to the weight of each animal.
Chemotherapy
The non-specific cytotoxic agent cisplatin/ hospira 100mg/100ml, ONCO-TAIN™, HOSPIRA UK, LIMITED was obtained from our pulmonary oncology department and it was administered according to the AUC calculation.
Nebulizer
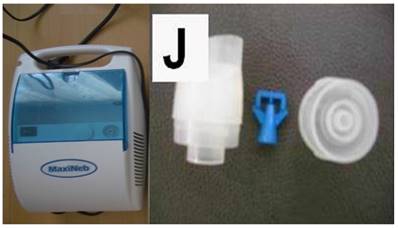
The jet nebulizer maxineb with the residual cup “J” was used for the administration of aerosol nivolumab and aerosol cisplatin based on our previous experiments.[24] Figure 1. (supplementary data attached to the submission)
Left figure jet-nebulizer MAXINEB, and right figure residual cup with the name “J”.
Aerosol Administration
The aerosol administration was performed with a specially designed cage that we have previously used in our experiments.[22, 25]
Study Protocol and Results
Based on our previous experiments and immunotherapy function we designed our current experiment to last for ten weeks. Our main concern was the application of the immunotherapy post or prior to cisplatin. Since there are data demonstrating that immunotherapy can have an either if it is administered prior to immunotherapy or after.[26, 27] Moreover; we did not want to have increased adverse effects from the therapy combination so we chose to have two groups with the combination treatment firstly administer cisplatin prior to immunotherapy with a delay of three days and an additional group administration of immunotherapy prior to cisplatin with a delay of three days.
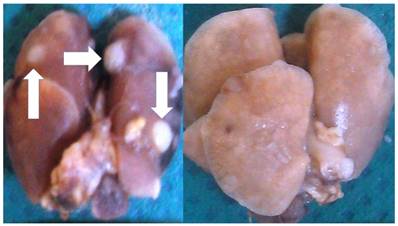
Initiation of the experiment was done when the tumor volume reached 100mm3, this was observed after two weeks of the cancer line inoculation. In the immunotherapy group the administration was performed every two weeks and in the cisplatin group every 20 days. Regarding the other two groups again we chose to keep the previous mentioned timeline therapy administration. Groups: a) Control Group, B) Immunotherapy Group, c) Cisplatin Group, d) cisplatin prior to immunotherapy with a delay of three days and d) administration of immunotherapy prior to cisplatin with a delay of three days. Median survival: Group A) 15 days, Group B) 42 days, Group C) 39 days, Group D) 58 days and Group E) 51 days. In the control group most of the mice had lung cancer metastasis upon death. In Groups D and E only 7 mice had lung metastasis in total 3/4, however; 12 had liver and peritoneal metastasis 5/7. Figure 2.
Left figure; multiple lung metastasis (white arrows) from a BALBC mice from the control group and right figure lung upon death from a BALBC mouse from group D.
Discussion
Immunotherapy with the drug nivolumab has a license for now as second line treatment since data of Carbone D.P. et. al. [28] for first line treatment did not present significant results in order for nivolumab to be administered as first line treatment. Pembrolizumab on the other hand has license as first line treatment if the PD-L1 expression is >50% and as second line treatment if PD-L1 expression is > 2%. Nowadays there are ongoing studies were immunotherapy combinations are being studied and moreover; chemotherapy plus immunotherapy. [29-31] Another issue that has to be addressed is whether radiotherapy treatment prior to immunotherapy administration has a favorable synergistic effect for immunotherapy.[32] Indeed current data indicate that administration of radiotherapy enhance immunotherapy treatment, however; the time and dose of radiotherapy administration are yet to be clarified.[33, 34] Radiotherapy is unique as a partner for combination treatments in that it is readily available, unrestricted by patent rights, and part of the standard of care for almost every cancer. The contribution of T cells to radiation-induced tumor control was shown in mouse models over thirty years ago and, more recently, the availability of T cell receptor (TCR)-transgenic mice made it possible to unequivocally demonstrate that radiation can induce priming of T cells to exogenous “model” antigens expressed by tumors. These studies, together with the demonstration that radiation induces immunogenic cell death, have provided proof of principle evidence that radiation can induce tumor-specific T cells.[35-39] The same principle more or less is for chemotherapy. Chemotherapy kills tumor cells, those cells release molecules that can attract T cells to join in the attack. Previous studies presented data where patients who received standard chemotherapy (carboplatin or paclitaxel) before surgery there was significantly more activation of the kind of T cells that attack cancer cells than in the other patients not receiving chemotherapy. It was observed that chemotherapy suppresses the immune system, but weeks after treatment, a large number of T cells was found in the tumor environment.
Just having the T cells clearly isn't enough. It has been observed that surviving tumor cells were disabling the swarming T cells. But immunotherapy such as Merck's pembrolizumab and Bristol-Myers Squibb's ipilimumab can stop tumor cells from doing that. The principle is that a cold tumor like cancer might be turned into a hot, T cell-attracting one and therefore the immunotherapy will become more effective.[40-42]
Major limitation of our study was the unknown status of PD-L1 which plays a crucial role in immunotherapy and furthermore we do not have the status of the tumor mutation burden which plays a crucial role for the efficiency of nivolumab.[28] Based on recent publications we had to decide which was the appropriate time of chemotherapy application and therapy toxicity. Recent studies indicate that chemotherapy administration prior to immunotherapy enhances the immunotherapy treatment, but also chemotherapy treatment after immunotherapy has efficient therapeutic effect.[27, 43] We chose to investigate the treatment schedules. Another challenge was the time of administration which there are no data or suggestions. We chose to administer immunotherapy after three days of the chemotherapy application based on the current knowledge that the drug cisplatin circulates within the body for less than 24 hours and therefore the cytotoxicity effect has already began and it is on its peek at the time of the immunotherapy application. On the other hand certainly immunotherapy has a slower rhythm of efficiency and therefore possibly the application of chemotherapy should be later than the time that we chose. However; at the same time we have to consider the tumor burden and indeed the survival of group E was close to group D. Another limitation of our study was that we did not acquire blood samples in order to check cancer antigen populations and therefore treatment efficiency with this method. In a future experiment of ours we will challenge the time of chemotherapy administration after immunotherapy. Another issue that we have to consider is the different circadian rhythm and hormones that animals have versus humans which also might play a crucial role for immunotherapy.[44]
Competing Interests
The authors have declared that no competing interest exists.
References
1. Haidong H, Yunye N, Wei Z, Zarogoulidis P, Hohenforst-Schmidt W, Man YG. et al. Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions: a single-center study. Journal of Cancer. 2017;8:3514-21 doi:10.7150/jca.20035
2. Zaric B, Stojsic V, Carapic V, Kovacevic T, Stojanovic G, Panjkovic M. et al. Radial Endobronchial Ultrasound (EBUS) Guided Suction Catheter-Biopsy in Histological Diagnosis of Peripheral Pulmonary Lesions. Journal of Cancer. 2016;7:7-13 doi:10.7150/jca.13081
3. Zarogoulidis P, Huang H, Bai C, Kosmidis C, Trakada G, Veletza L. et al. Endobronchial ultrasound convex probe for lymphoma, sarcoidosis, lung cancer and other thoracic entities. A case series. Respiratory medicine case reports. 2017;22:187-96 doi:10.1016/j.rmcr.2017.08.016
4. Darwiche K, Zarogoulidis P, Baehner K, Welter S, Tetzner R, Wohlschlaeger J. et al. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24:2866-70 doi:10.1093/annonc/mdt365
5. Oezkan F, Khan A, Zarogoulidis P, Hohenforst-Schmidt W, Theegarten D, Yasufuku K. et al. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. OncoTargets and therapy. 2014;7:2061-5 doi:10.2147/OTT.S72974
6. Zarogoulidis P, Huang H, Bai C, Kosmidis C, Porpodis K, Kallianos A. et al. A new mode of ventilation for interventional pulmonology. A case with EBUS-TBNA and debulking. Respiratory medicine case reports. 2018;23:38-42 doi:10.1016/j.rmcr.2017.11.006
7. Nanavaty P, Alvarez MS, Alberts WM. Lung cancer screening: advantages, controversies, and applications. Cancer control: journal of the Moffitt Cancer Center. 2014;21:9-14 doi:10.1177/107327481402100102
8. Sadacca B, Hamy-Petit AS, Laurent C, Gestraud P, Bonsang-Kitzis H, Pinheiro A. et al. New insight for pharmacogenomics studies from the transcriptional analysis of two large-scale cancer cell line panels. Scientific reports. 2017;7:15126. doi:10.1038/s41598-017-14770-6
9. Kallianos A, Rapti A, Zarogoulidis P, Tsakiridis K, Mpakas A, Katsikogiannis N. et al. Therapeutic procedure in small cell lung cancer. Journal of thoracic disease. 2013;5(Suppl 4):S420-4 doi:10.3978/j.issn.2072-1439.2013.09.16
10. Zarogoulidis K, Ziogas E, Boutsikou E, Zarogoulidis P, Darwiche K, Kontakiotis T. et al. Immunomodifiers in combination with conventional chemotherapy in small cell lung cancer: a phase II, randomized study. Drug design, development and therapy. 2013;7:611-7 doi:10.2147/DDDT.S43184
11. Zarogoulidis K, Latsios D, Porpodis K, Zarogoulidis P, Darwiche K, Antoniou N. et al. New dilemmas in small-cell lung cancer TNM clinical staging. OncoTargets and therapy. 2013;6:539-47 doi:10.2147/OTT.S44201
12. Zarogoulidis K, Eleftheriadou E, Kontakiotis T, Gerasimou G, Zarogoulidis P, Sapardanis I. et al. Long acting somatostatin analogues in combination to antineoplastic agents in the treatment of small cell lung cancer patients. Lung cancer. 2012;76:84-8 doi:10.1016/j.lungcan.2011.09.014
13. Zarogoulidis K, Boutsikou E, Zarogoulidis P, Darwiche K, Freitag L, Porpodis K. et al. The role of second-line chemotherapy in small cell lung cancer: a retrospective analysis. OncoTargets and therapy. 2013;6:1493-500 doi:10.2147/OTT.S52330
14. Pinquie F, Goupil F, Oster JP, Dixmier A, Renault PA, Levy A. et al. [Therapeutic strategies in patients undergoing surgery for non-small cell lung cancer. Results of the ESCAP-2011-CPHG study, promoted by the French College of General Hospital Respiratory Physicians (CPHG)]. Revue des maladies respiratoires. 2017;34:976-90 doi:10.1016/j.rmr.2017.05.002
15. Shojaee S, Nana-Sinkam P. Recent advances in the management of non-small cell lung cancer. F1000Research. 2017;6:2110. doi:10.12688/f1000research.11471.1
16. Yi JS, Ready N, Healy P, Dumbauld C, Osborne R, Berry M. et al. Immune Activation in Early-Stage Non-Small Cell Lung Cancer Patients Receiving Neoadjuvant Chemotherapy Plus Ipilimumab. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23:7474-82 doi:10.1158/1078-0432.CCR-17-2005
17. Pitsiou G, Zarogoulidis P, Petridis D, Kioumis I, Lampaki S, Organtzis J. et al. Inhaled tyrosine kinase inhibitors for pulmonary hypertension: a possible future treatment. Drug design, development and therapy. 2014;8:1753-63 doi:10.2147/DDDT.S70277
18. Zarogoulidis P, Porpodis K, Kioumis I, Petridis D, Lampaki S, Spyratos D. et al. Experimentation with inhaled bronchodilators and corticosteroids. International journal of pharmaceutics. 2014;461:411-8 doi:10.1016/j.ijpharm.2013.12.010
19. Zarogoulidis P, Petridis D, Ritzoulis C, Li Q, Huang H, Ning Y. et al. Further experimentation of inhaled; LANTUS, ACTRAPID and HUMULIN with todays' production systems. International journal of pharmaceutics. 2013;458:39-47 doi:10.1016/j.ijpharm.2013.10.019
20. Zarogoulidis P, Kioumis I, Porpodis K, Spyratos D, Tsakiridis K, Huang H. et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug design, development and therapy. 2013;7:1115-34 doi:10.2147/DDDT.S51303
21. Zarogoulidis P, Papanas N, Kouliatsis G, Spyratos D, Zarogoulidis K, Maltezos E. Inhaled insulin: too soon to be forgotten? Journal of aerosol medicine and pulmonary drug delivery. 2011;24:213-23 doi:10.1089/jamp.2011.0876
22. Zarogoulidis P, Hohenforst-Schmidt W, Darwiche K, Krauss L, Sparopoulou D, Sakkas L. et al. 2-diethylaminoethyl-dextran methyl methacrylate copolymer nonviral vector: still a long way toward the safety of aerosol gene therapy. Gene therapy. 2013;20:1022-8 doi:10.1038/gt.2013.27
23. Bertram JS, Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63-73 doi:0304-3835(80)90130-5 [pii]
24. Hohenforst-Schmidt W, Zarogoulidis P, Linsmeier B, Kioumis I, Li Q, Huang H. et al. Enhancement of Aerosol Cisplatin Chemotherapy with Gene Therapy Expressing ABC10 protein in Respiratory System. Journal of Cancer. 2014;5:344-50 doi:10.7150/jca.9021
25. Zarogoulidis P, Hohenforst-Schmidt W, Huang H, Sahpatzidou D, Freitag L, Sakkas L. et al. A gene therapy induced emphysema model and the protective role of stem cells. Diagnostic pathology. 2014;9:195. doi:10.1186/s13000-014-0195-7
26. Zhao M, Li H, Li L, Zhang Y. Effects of a gemcitabine plus platinum regimen combined with a dendritic cell-cytokine induced killer immunotherapy on recurrence and survival rate of non-small cell lung cancer patients. Experimental and therapeutic medicine. 2014;7:1403-7 doi:10.3892/etm.2014.1574
27. Dwary AD, Master S, Patel A, Cole C, Mansour R, Mills G. et al. Excellent response to chemotherapy post immunotherapy. Oncotarget. 2017;8:91795-802 doi:10.18632/oncotarget.20030
28. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M. et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. The New England journal of medicine. 2017;376:2415-26 doi:10.1056/NEJMoa1613493
29. Saka H, Kitagawa C, Ichinose Y, Takenoyama M, Ibata H, Kato T. et al. A randomized phase II study to assess the effect of adjuvant immunotherapy using alpha-GalCer-pulsed dendritic cells in the patients with completely resected stage II-IIIA non-small cell lung cancer: study protocol for a randomized controlled trial. Trials. 2017;18:429. doi:10.1186/s13063-017-2103-4
30. Weiss GJ, Waypa J, Blaydorn L, Coats J, McGahey K, Sangal A. et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). British journal of cancer. 2017;117:33-40 doi:10.1038/bjc.2017.145
31. Popat S, Mellemgaard A, Fahrbach K, Martin A, Rizzo M, Kaiser R. et al. Nintedanib plus docetaxel as second-line therapy in patients with non-small-cell lung cancer: a network meta-analysis. Future oncology. 2015;11:409-20 doi:10.2217/fon.14.290
32. Krcik EM. Radiation Therapy Plus Anti-Programmed Death Ligand 1 Immunotherapy: A Review on Overall Survival. Radiologic technology. 2016;88:123-8
33. Luterstein E, Shaverdian N, Lee P. Radiotherapy: the key to immunotherapy ignition? Oncotarget. 2017;8:93307-8 doi:10.18632/oncotarget.22070
34. Takamori S, Toyokawa G, Takada K, Shoji F, Okamoto T, Maehara Y. Combination Therapy of Radiotherapy and Anti-PD-1/PD-L1 Treatment in Non-Small-cell Lung Cancer: A Mini-review. Clinical lung cancer. 2017 doi:10.1016/j.cllc.2017.06.015
35. Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. Journal of the National Cancer Institute. 1979;63:1229-35
36. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. Journal of immunology. 2005;174:7516-23
37. Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589-95 doi:10.1182/blood-2009-02-206870
38. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine. 2007;13:1050-9 doi:10.1038/nm1622
39. Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Seminars in radiation oncology. 2015;25:11-7 doi:10.1016/j.semradonc.2014.07.005
40. Prendergast GC, Mondal A, Dey S, Laury-Kleintop LD, Muller AJ. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive 'Cold' Tumors 'Hot'. Trends in cancer. 2018;4:38-58 doi:10.1016/j.trecan.2017.11.005
41. Haanen J. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell. 2017;170:1055-6 doi:10.1016/j.cell.2017.08.031
42. Wu A, Zhang Q, Lambert G, Khin Z, Gatenby RA, Kim HJ. et al. Ancient hot and cold genes and chemotherapy resistance emergence. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10467-72 doi:10.1073/pnas.1512396112
43. Perez-Gracia JL, Loriot Y, Rosenberg JE, Powles T, Necchi A, Hussain SA. et al. Atezolizumab in Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: Outcomes by Prior Number of Regimens. European urology. 2017 doi:10.1016/j.eururo.2017.11.023
44. Zarogoulidis P, Darwiche K, Huang H, Spyratos D, Yarmus L, Li Q. et al. Time recall; future concept of chronomodulating chemotherapy for cancer. Current pharmaceutical biotechnology. 2013;14:632-42
Author contact
![]() Corresponding author: Paul Zarogoulidis, M.D, Ph.D, Pulmonary Oncology-Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece. E-mail: pzarogcom; and Konstantinos Sapalidis, M.D, Ph.D, 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece. E-mail: sapalidiskonstantinoscom
Corresponding author: Paul Zarogoulidis, M.D, Ph.D, Pulmonary Oncology-Unit, “Theageneio” Cancer Hospital, Thessaloniki, Greece. E-mail: pzarogcom; and Konstantinos Sapalidis, M.D, Ph.D, 3rd Department of Surgery, “AHEPA” University Hospital, Aristotle University of Thessaloniki, Medical School, Thessaloniki, Greece. E-mail: sapalidiskonstantinoscom

 Global reach, higher impact
Global reach, higher impact