3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(7):1214-1222. doi:10.7150/jca.18707 This issue Cite
Research Paper
Elevated preoperative neutrophil-to-lymphocyte ratio can predict poor survival in early stage gastric cancer patients receiving radical gastrectomy: The Fujian prospective investigation of cancer (FIESTA) study
1. Department of Pathology, Fujian Provincial Cancer Hospital, The Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China;
2. Department of Medical Record, Fujian Provincial Cancer Hospital, The Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China;
3. Department of Clinical Laboratory, Fujian Provincial Cancer Hospital, The Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China;
4. Department of Cardiology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China;
5. State Key Laboratory of Medical Genomics, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
*Co- first authors.
Received 2016-12-12; Accepted 2017-2-8; Published 2017-4-10
Abstract
Aims: This cohort study was conducted to evaluate the prognostic impact of blood-routine parameters before radical gastrectomy on gastric cancer mortality.
Methods: Total 3012 patients with gastric cancer were consecutively enrolled from a mono-center between 2000 and 2010, and the latest follow-up was completed in 2015.
Results: The median follow-up time was 44.05 months. Finally, 1331 out of 3012 gastric cancer patients died from gastric cancer. Per standard deviation increment in neutrophil (hazard ratio or HR=1.08, P<0.001), white blood cell count (HR=1.07, P=0.001), neutrophil-to-lymphocyte ratio or NLR (HR=1.08, P<0.001) and platelet-to-lymphocyte ratio (HR=1.08, P<0.001) was significantly associated with an increased risk of gastric cancer mortality, while that in lymphocyte (HR=0.69, P<0.001), hemoglobin (HR=0.82, P<0.001) and lymphocyte-to-monocyte ratio (HR=0.68, P<0.001) was associated with a reduced risk. Survival tree analysis indicated that in patients with TNM stage I/II, the contrasts of NLR>2.61 with ≤2.61 and NLR>1.87 with ≤1.87 were respectively associated with a 5.21-fold (P=0.004) and 2.36-fold (P=0.001) increased risk of gastric cancer mortality. The effect-size magnitude of NLR was further potentiated in patients with invasion depth T1/T2 (HR=1.73, P=0.001), regional lymph node metastasis N0 (HR=1.60, P<0.001), TNM stage I/II (HR=1.36, P=0.009) and tumor size ≤ 4.5 cm (HR=1.17, P<0.001).
Conclusions: Our findings consolidated the prognostic impact of preoperative NLR on gastric mortality, and demonstrated that elevated preoperative NLR was a robust indicator of poor survival in patients at early stage.
Keywords: Gastric cancer, Blood-routine parameter, Prognosis, Survival.
Introduction
Gastric cancer tops the incidence of malignancies and is the leading cause of cancer mortality worldwide [1]. In China, it is projected that 679 100 new cases and 498 000 deaths from gastric cancer occurred during the year 2015 [2]. A long-existing dilemma facing clinical doctors is that the majority of patients with gastric cancer are diagnosed at high-grade invasive stages, mainly due to the lack of obvious symptoms, clinical indications and effective surveillance programs. Moreover, the overall 5-year survival rate of gastric cancer revolves around 20% in China [3]. An effective target for early identification and intervention of gastric cancer will be high on the agenda. One of the most urgent tasks before us is to seek some prognostic biomarkers that can assist in improving the clinical management of gastric cancer patients.
Recent two decades have witnessed some major achievements in identifying gastric cancer-specific biomarkers in scientific research and medical applications; however, no general agreement exists yet as to the clinical benefits of any single biomarkers for the diagnosis, prognosis and therapy of gastric cancer [4-8]. One of possible reasons is that most conclusions in current literature are based on underpowered studies that are prone to false-positive errors [9]. Besides, diversity in population characteristics such as dietary habits and lifestyles may also add up. For instance, high-sodium diets and cigarette smoking are established as major contributing factors for the development of gastric cancer [10-12]. For practical reasons, a growing number of studies have interrogated the association of blood-routine parameters with the risk and prognosis of gastric cancer [13-16]. It is worth mentioning that the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are widely evaluated and their prognostic capability for high-risk gastric cancer has been verified in many cohort studies [17-19]. In spite of exhaustive research regarding this subject, it still remains elusive whether the prognostic impact of these significant parameters on gastric cancer varies across distinct clinicopathologic settings. Further optimizing the cutoff value(s) of significant parameter(s) poses a formidable challenge. A traditional method to determine an optimal cutoff value relies on the receiver-operator characteristic analysis, while its incapability to consider survival time hampers the discrimination utility, leading to an inaccurate classification. To overcome this limitation and yield more information, we aimed to evaluate the prognostic impact of blood-routine parameters before radical gastrectomy on gastric cancer mortality among 3012 patients over a 15-year follow-up period by eliciting a subset of the data from the ongoing Fujian prospective investigation of cancer (FIESTA) study [20-24]. In addition, we adopted survival tree analysis to determine the optimal cutoff value of the most significant parameter and further test its prognostic specificity across patients within different clinicopathologic profiles.
Methods
Study patients
All study patients were obtained from the ongoing FIESTA study initiated in January 2000. The FIESTA study is an ongoing prospective cohort study of common digestive system tumors, including esophageal cancer, gastric cancer and colorectal cancer [20-24], and the chief objective of the FIESTA study is attempting to look for preoperative risk factors for cancer-specific mortality, which will assist in defining rational targets to delay tumor progression and prolong the survival of cancer patients after surgery. For this study, we consecutively collected preoperative patients with operable gastric cancer until December 2010 from the Department of Thoracic Surgery, Fujian Provincial Cancer Hospital. The latest follow-up was finished in December 2015. All study patients gave written informed consent to denoting blood and tissue samples for clinical assays and to being contacted during follow-up. The ethical committees of Fujian Provincial Cancer Hospital approved this study. All study protocols were implemented in accordance with the relevant guidelines and regulations.
Tissue collection and diagnosis
Each patient agreed to provide a pair of cancer and normal tissue samples, which were immediately fixed by 10% neutral formalin and embedded in paraffin. The clinicopathologic characteristics were determined at the Department of Pathology, Fujian Provincial Cancer Hospital.
Eligibility criteria
Eligible gastric cancer patients must simultaneously meet the following criteria: (i) they were of Han Chinese nationality; (ii) they had no consanguinity at enrollment; (iii) they were firstly hospitalized for radical gastrectomy; (iv) they received no preoperative/postoperative chemotherapy or radiotherapy; (v) they must be followed up for 1 month and more; (vi) they must have gastric cancer confirmed by preoperative biopsy or postoperative pathologic tests.
Follow-up assessment
After radical gastrectomy, all patients were requested to be rechecked every six months at the Out-Patient Department, Fujian Provincial Cancer Hospital, and in case of no-show at the reserved time they were contacted through calling or sending post mails. We followed each patient up from the initial enrollment date to death time or the last checking point during 2015, whichever came first, and survival period was transformed into months for survival analysis.
Blood-routine parameters
In the morning of surgery day, fasting 4 mL venous blood samples were collected into an EDTA-K2 anticoagulative tube for measuring blood-routine parameters, including neutrophil, lymphocyte, monocyte, eosinophil, basophil, white blood cell count, red blood cell count, hemoglobin, red cell distribution width and platelet count by the SYSMEX XE-2100 Automatic Blood Cell Analyzer (Sysmex, Kobe, Japan) at the Clinical Laboratory, Fujian Provincial Cancer Hospital. The period from blood drawing to clinical analysis was less than 4 hours according to a standard procedure. In addition, based on single blood-routine parameters, we calculated NLR, PLR and lymphocyte-to-monocyte ratio (LMR) accordingly.
Demographic and clinicopathologic characteristics
At the time of enrollment, we invited each patient to complete a self-designed questionnaire to record demographic information, including date of birth, age of onset for gastric cancer, gender, body weight, body height, smoking, drinking and family cancer history. Age was defined as the age at the time of operation for gastric cancer. Body mass index was calculated as weight (in kilograms) divided by the square of height (in meters). Smoking status was classified as never smoking and ever smoking (including formerly and currently smoking). Drinking status was classified as never drinking and ever drinking (including formerly and currently drinking).
Clinicopathologic data were gathered immediately after radical gastrectomy from medical charts and pathological reports, including tumor-node-metastasis (TNM) stage (I, II, III and IV according to the 7th Edition of the UICC/AJCC TNM Staging System [25]) , tumor size (in centimeters), depth of invasion (T1, T2, T3 and T4), regional lymph node metastasis (N0, N1, N2 and N3), distant metastasis (M0 and M1), the Lauren's classification (intestinal type and diffuse type) and embolus (positivity and negativity).
Statistical analysis
Continuous variables expressed as median (inter-quartile range) were compared between non-survivors and survivors by the Mann-Whitney U test, and categorical variables expressed as percentage were compared by the Chi-squared test. Both continuous and categorical variables were analyzed as their original forms in the following analyses. The Kaplan-Meier method was used to estimate survival time and the Log-rank test was used for survival comparisons. The prediction of blood-routine parameters for gastric cancer mortality was expressed as hazard ratio (HR) and its 95% confidence interval (95% CI) by using the multivariate Weibull proportional hazards regression model. The STREE program (http://c2s2.yale.edu/software/stree/) was adopted to determine the optimal cutoff values by splitting patients with significantly differed median survival time (MST). Unless otherwise stated, all statistical tests are two-sized and are significant at a level of 5%, and the STATA software (StataCorp, TX, USA, version 13.0) was used for statistical analysis.
Results
Baseline characteristics
In total, this study involved 3413 gastric cancer patients. Due to the loss to follow-up (n=318), failure to survive over 1 month (n=48) and death of causes other than gastric cancer (n=35), we finally had 3012 gastric cancer patients with complete data for survival analysis. The follow-up time ranged from 1.1 to 183.3 months and the median was 44.05 months. As of late 2015, 1331 out of 3012 gastric cancer patients were recorded to die from gastric cancer.
The comparisons of demographic and clinicopathologic characteristics, as well as blood-routine parameters are shown in Table 1. Non-survivors were older than survivors at enrollment (median: 60 years vs. 58 years, P<0.001). In addition, baseline body mass index was higher in non-survivors than in survivors (P=0.007). There were no differences in male percentage (P=0.144) between the two groups, as well as in the percentages of smoking (P=0.212), drinking (P=0.643) and family cancer history (P=0.214). The median levels of neutrophil, white blood cell count, NLR and PLR were significantly higher in non-survivors than survivors, while that of lymphocyte, hemoglobin and LMR were significantly lower (P<0.05). No significance difference was noted for the other blood-routine parameters under study. Further for clinicopathologic characteristics, the distributions of invasion depth, regional lymph node metastasis, distant metastasis, TNM stage, Lauren's classification and embolus differed significantly between the two groups (P<0.001 for all).
Prediction of blood-routine parameters
The risk effect-size estimates of blood-routine parameters for gastric cancer mortality per standard deviation (SD) increment are summarized in Table 2. Considering the facts that the mortality rate of gastric cancer increases smoothly over time and the ln(-ln(S(t))) is a linear function of ln(t) (here, t is survival time, and S(t) is survival function), it is more appropriate to adopt the Weibull proportional hazards model for multivariate-adjusted survival analysis. As compared with the crude results, we controlled for age, gender, body mass index, smoking, drinking and family-cancer history under the multivariate Weibull proportional hazards regression model. Per SD increment in neutrophil (adjusted HR, 95% CI, P: 1.08, 1.05-1.11, <0.001), white blood cell count (1.07, 1.03-1.11, 0.001), NLR (1.08, 1.06-1.12, <0.001) and PLR (1.08, 1.05-1.11, <0.001) was associated with a significantly increased risk for gastric cancer mortality, respectively, even after adjusting for confounding factors, while per SD increment in lymphocyte (0.69, 0.62-0.77, <0.001), hemoglobin (0.82, 0.78-0.87, <0.001) and LMR (0.68, 0.59-0.80, <0.001) was significantly associated with a reduced risk. A marginally increased risk was noted for platelet count (1.06, 1.00-1.13, 0.036).
The baseline characteristics of cohort patients by primary clinical outcome
| Characteristics | Non-survivors (n=1331) | Survivors (n=1681) | P |
|---|---|---|---|
| Age (years) | 60 (52, 68) | 58 (51, 66) | <0.001 |
| Males | 73.03% | 75.37% | 0.144 |
| Body mass index (kg/m2) | 22.72 (20.63, 25.21) | 22.5 (20.70, 24.44) | 0.007 |
| Smoking | 18.96% | 20.87% | 0.212 |
| Drinking | 5.82% | 6.24% | 0.643 |
| Family cancer history | 8.53% | 9.91% | 0.214 |
| Neutrophil (109/L) | 3.9 (3.0, 5.0) | 3.6 (2.7, 4.6) | <0.001 |
| Lymphocyte (109/L) | 1.7 (1.3, 2.1) | 1.9 (1.5, 2.3) | <0.001 |
| Monocyte (109/L) | 0.5 (0.4, 0.6) | 0.5 (0.4, 0.6) | 0.225 |
| Eosinophil (109/L) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | 0.128 |
| Basophil (109/L) | 0 (0, 0) | 0 (0, 0) | 0.043 |
| White blood cell count (109/L) | 6.4 (5.3, 7.7) | 6.3 (5.1, 7.6) | 0.005 |
| Red blood cell count (1012/L) | 4.1 (3.6, 4.48) | 4.3 (3.8, 4.62) | 0.914 |
| Hemoglobin (g/L) | 120 (98, 134) | 130 (111, 142) | <0.001 |
| Platelet count (109/L) | 253 (204, 317) | 251 (202, 307) | 0.059 |
| Red cell distribution width (%) | 13.4 (12.5, 15.3) | 13.1 (12.4, 14.2) | 0.169 |
| Neutrophil-to-lymphocyte ratio | 2.27 (1.70, 3.06) | 1.86 (1.38, 2.61) | <0.001 |
| Platelet-to-lymphocyte ratio | 151.3 (110.9, 204.9) | 133.7 (101.1, 180.0) | <0.001 |
| Lymphocyte-to-monocyte ratio | 3.3 (2.5, 4.3) | 3.8 (2.9, 5.0) | <0.001 |
| Invasion depth | <0.001 | ||
| T1 | 1.00% | 15.58% | |
| T2 | 3.69% | 13.38% | |
| T3 | 54.46% | 55.85% | |
| T4 | 40.85% | 15.19% | |
| Regional lymph node metastasis | <0.001 | ||
| N0 | 9.69% | 40.89% | |
| N1 | 30.31% | 32.43% | |
| N2 | 44.31% | 23.45% | |
| N3 | 15.69% | 3.23% | |
| Distant metastasis | |||
| Negative | 74.75% | 98.58% | <0.001 |
| Positive | 25.25% | 1.42% | |
| Tumor-node-metastasis stage | <0.001 | ||
| I | 1.23% | 21.20% | |
| II | 7.41% | 21.20% | |
| III | 59.34% | 54.75% | |
| IV | 32.02% | 2.84% | |
| The Lauren's classification | <0.001 | ||
| Intestinal type | 30.60% | 45.76% | |
| Diffuse type | 69.40% | 54.24% | |
| Embolus | <0.001 | ||
| Negative | 49.88% | 70.22% | |
| Positive | 50.12% | 29.78% | |
| Tumor size (cm) | 6.0 (4.5, 8.0) | 4.0 (3.0, 6.0) | <0.001 |
Data are expressed as median (interquartile range) or percentage. P was calculated by the Mann-Whitney U test or the Chi-squared test where appropriate.
The risk effect-size estimates of blood routine parameters for gastric cancer mortality
| Blood routine indexes | Increment (s. d.) | HR, 95% CI, P | HR, 95% CI, P* |
|---|---|---|---|
| Neutrophil (109/L) | 2.40 | 1.08, 1.05-1.11, <0.001 | 1.08, 1.05-1.11, <0.001 |
| Lymphocyte (109/L) | 1.08 | 0.67, 0.60-0.74, <0.001 | 0.69, 0.62-0.77, <0.001 |
| Monocyte (109/L) | 0.42 | 1.02, 0.97-1.07, 0.439 | 1.01, 0.97-1.07, 0.559 |
| Eosinophil (109/L) | 0.24 | 0.94, 0.87-1.01, 0.083 | 0.94, 0.87-1.01, 0.110 |
| Basophil (109/L) | 0.22 | 1.03, 0.98-1.07, 0.230 | 1.03, 0.99-1.07, 0.198 |
| White blood cell count (109/L) | 2.87 | 1.07, 1.03-1.11, 0.001 | 1.07, 1.03-1.11, 0.001 |
| Red blood cell count (1012/L) | 16.58 | 0.98, 0.91-1.07, 0.714 | 0.99, 0.91-1.07, 0.820 |
| Hemoglobin (g/L) | 28.73 | 0.82, 0.78-0.86, <0.001 | 0.82, 0.78-0.87, <0.001 |
| Platelet count (109/L) | 96.56 | 1.06, 1.00-1.12, 0.041 | 1.06, 1.00-1.13, 0.036 |
| Red cell distribution width (%) | 16.58 | 1.04, 0.99-1.09, 0.111 | 1.04, 0.99-1.09, 0.109 |
| Neutrophil-to-lymphocyte ratio | 2.63 | 1.09, 1.06-1.12, <0.001 | 1.08, 1.06-1.12, <0.001 |
| Platelet-to-lymphocyte ratio | 106.65 | 1.08, 1.05-1.11, <0.001 | 1.08, 1.05-1.11, <0.001 |
| Lymphocyte-to-monocyte ratio | 3.89 | 0.65, 0.56-0.75, <0.001 | 0.68, 0.59-0.80, <0.001 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval. The effect-size estimates were calculated under the Weibull proportional hazards regression models. *P was adjusted for age, gender, body mass index, smoking, drinking and family cancer history.
Risk classification of TNM stage based on survival tree analysis and the effect-size estimates for gastric cancer mortality
| TNM stage | Comparison | HR, 95% CI, P | HR, 95% CI, P* |
|---|---|---|---|
| I | NLR: >2.61 vs. ≤2.61 | 8.48, 3.02-23.83, <0.001 | 5.21, 1.71-15.90, 0.004 |
| II | NLR: >1.87 vs. ≤1.87 | 2.76, 1.74-4.38, <0.001 | 2.36, 1.45-3.85, 0.001 |
| III | LNM: >9 vs. ≤9 | 2.43, 2.08-2.83, <0.001 | 2.51, 2.12-2.98, <0.001 |
| IV | Tumor size (cm): >4.5 vs. ≤4.5 | 1.81, 1.39-2.36, <0.001 | 1.75, 1.33-2.31, <0.001 |
Abbreviations: TNM, tumor node metastasis; NLR, neutrophil-to-lymphocyte ratio; LNM, the number of regional lymph node metastasis; HR, hazard ratio; 95% CI, 95% confidence interval. The effect-size estimates were calculated under the Weibull proportional hazards regression models. *P was adjusted for age, gender, body mass index, smoking, drinking and family cancer history.
Survival tree analysis
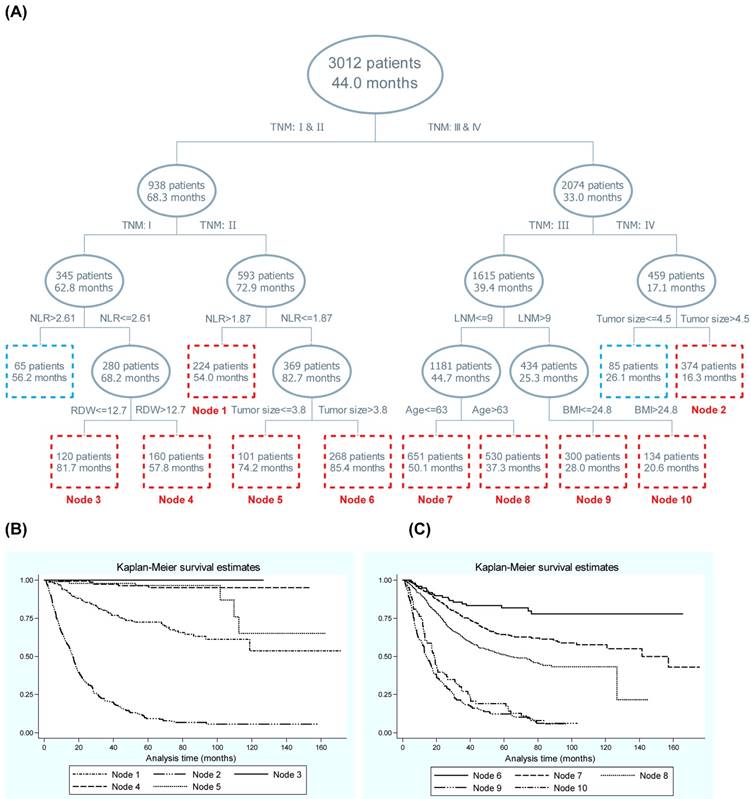
Displayed in Figure 1 is the survival tree structure over blood-routine parameters, demographic and clinicopathologic characteristics. The top splitting factor was TNM stage, and the MST in patients with TNM stage III-IV was half of that in patients with TNM stage I-II (33.0 months vs. 68.3 months, P<0.001). The discrimination ability of NLR was secondary to that of TNM in both patients with stage I and II. In contrast to patients with TNM stage III and IV, regional lymph node metastasis and tumor size served as the second splitting factors, respectively. To enhance statistical power, only nodes involving at least 100 patients were numbered and a total of 10 nodes were selected, and their discrimination ability for gastric cancer mortality was further embodied in the Kaplan-Meier curves (Figure 1).
Given the importance of TNM stage in survival tree analysis, we calculated the risk effect-size estimates of the secondary splitting factor within each stage (Table 3). In patients with TNM stage I and II, the contrast of NLR >2.61 with ≤2.61 and NLR >1.87 with ≤1.87 was respectively associated with a 5.21-fold (adjusted HR, 95% CI, P: 5.21, 1.71-15.90, 0.004) and 2.36-fold (2.36, 1.45-3.85, 0.001) increased risk of gastric cancer mortality after adjusting for confounders. In addition for patients with TNM stage III and IV, the contrast of regional lymph node metastasis number >9 with ≤9 and tumor size >4.5 cm with ≤4.5 cm was respectively with a 2.51-fold (2.51, 2.12-2.98, <0.001) and 1.75-fold (1.75, 1.33-2.31, <0.001) increased risk after adjustment.
Subgroup analysis
In view of the significant discrimination ability of NLR in survival tree analysis, we further examined its risk effect-size estimates per SD increment for gastric cancer mortality according to clinicopathologic characteristics, as shown in Table 4. The magnitude of effect-size estimates of NLR was potentiated in patients with invasion depth T1-T2 (adjusted HR, 95% CI, P: 1.73, 1.25-2.40, 0.001), regional lymph node metastasis N0 (1.60, 1.34-1.90, <0.001), TNM stage I-II (1.36, 1.08-1.70, 0.009), positive embolus (1.19, 1.11-1.28, <0.001) and tumor size ≤4.5 cm (1.17, 1.07-1.28, <0.001) relative to patients with invasion depth T3-T4, regional lymph node metastasis N1-N3, TNM stage III-IV, negative embolus and tumor size >4.5 cm, respectively. In addition for the Lauren's classification, the multivariate HRs were 1.18 (95% CI, P: 1.12-1.25, <0.001) and 1.06 (1.01-1.12, 0.026) for the diffuse and intestinal types, respectively. No difference was noticed for patients with positive and negative metastases.
Survival tree structure of blood-routine parameters along with demographic and clinicopathological characteristics. Abbreviations: NLR, Neutrophil-to-lymphocyte ratio; LNM, lymph node metastasis; RDW, red cell distribution width; BMI, body mass index. Panel B and C are the Kaplan-Meier curves of different nodes generated by survival tree analysis (panel A). In panel A, the upper number in the box refers to the number of patients, and the lower number refers to the median survival time.
Discussion
Via a 15-year prospective analysis of preoperative blood-routine parameters for the mortality of 3012 patients, our findings consolidated the prognostic value of preoperative NLR and demonstrated that elevated NLR was a robust indicator of poor survival in patients with early stage gastric cancer receiving radical gastrectomy. In addition, we adopted survival tree analysis and further optimized the discrimination accuracy of NLR by recommending cutoff values in patients with TNM stage I and II. To the best of our knowledge, this is to date the largest cohort study that has evaluated the prognostic significance of blood-routine parameters before the operation to predict gastric cancer mortality.
A recent meta-analysis by Sun et al who pooled the results of 19 studies and 5431 patients indicated that elevated pretreatment NLR was a significant predictor of poor outcomes for gastric cancer patients, particularly at late stage [17]. Consistent with the findings of this meta-analysis [17], we in an analysis of 3012 gastric cancer patients for radical gastrectomy reported that per SD increment in preoperative NLR was significantly associated with an 8% increased risk of gastric cancer mortality, independent of age, gender, obesity, smoking and drinking habits. It is also worth noting that, inconsistent with the meta-analytical findings by Sun et al [17], our subgroup analysis and survival tree analysis demonstrated that the discrimination ability of NLR for postoperative mortality was strongly potentiated in gastric cancer patients at TNM stage I and II rather than at stage III and IV, as well as in patients with low depth of invasion, no detectable lymph node metastasis and small tumor size, respectively. The major reason for this inconsistency may be due to the divergent lengths of the follow-up period. In the current cohort study, we consecutively enrolled gastric cancer patients for surgical treatment from 2000 to 2010 at the Fujian Provincial Cancer Hospital, and the latest follow-up was completed in late 2015. Such a long follow-up period allowed us to accurately assess the clinical outcomes of most gastric cancer patients in this study. Over 15-year follow-up, we found that 415 (90.41%) out of 459 patients with TNM stage IV died of gastric cancer. By contrast to the meta-analysis by Sun et al [17], the median follow-up time ranged from 9.3 months [26] to 96 months [27] and it is possible that in studies with a shorter follow-up period, the clinical outcomes of gastric cancer patients at advanced stage haven't happened yet by the end of follow-up, causing an overestimation of the true effect of NLR in prognosis. In addition, differences in age, gender composition, treatment modality, tumor size and tumor differentiation might also introduce a selection bias in the Sun et al meta-analysis [17]. We agree that confirmation of our prospective findings in another independent large cohort is critical. Nevertheless, considering the potential implications of preoperative NLR in predicting mortality, we maintain that the evaluation of blood-routine parameters for gastric cancer patients receiving radical gastrectomy, especially at an early stage, will facilitate risk stratification and the identification of high-risk patients for targeted preventative and therapeutic interventions.
Extending previous findings on this topic, we additionally recommended the optimal cutoff values for preoperative NLR in patients with early stage gastric cancer by employing survival tree analysis, and the prognostic utility of these cutoff values was further validated by the Weibull proportional hazards regression model (Table 3). Moreover, what has attracted our attention most in survival tree analysis is that the prognostic utility of NLR in gastric cancer patients with TNM stage I-II is as fairly important as that of regional lymph node metastasis and tumor size respectively in patients with TNM stage III and IV, highlighting the robustness of NLR as a early prognostic indicator for gastric cancer mortality. Although the exact mechanism behind this prognostic significance of NLR remains to be elucidated, it has been proposed that the dominant pro-tumor activities of neutrophils and reduced anti-tumor immune response by lymphocytes, as denoted by NLR, may have an impact on poor tumor response and unfavorable prognosis [28-30]. As supported by several lines of evidence, neutrophilia is the most sensitive responder to the inflammatory activity of the tumor, and elevated neutrophils in circulation can promote tumor growth and metastasis or suppress lymphocyte activity, thereby counteracting the antitumor immune response [16, 31-33]. In contrast, lymphocytopenia can reflect the depression of innate cellular immunity indicated by a marked decrease in T4 helper lymphocytes and an increase in T8 suppressor lymphocytes [34]. As such, it is reasonable to speculate that elevated NLR might, if involved, induce the inflammation/immune response and anti-tumor activities to be associated with poor prognosis of gastric cancer patients.
The effect-size estimates of neutrophil-to-lymphocyte ratio for gastric cancer mortality according to clinicopathological characteristics
| Subgroups | HR, 95% CI, P | HR, 95% CI, P* |
|---|---|---|
| Invasion depth | ||
| T1-T2 | 1.66, 1.24-2.23, 0.001 | 1.73, 1.25-2.40, 0.001 |
| T3-T4 | 1.07, 1.04-1.10, <0.001 | 1.07, 1.03-1.10, <0.001 |
| Regional lymph node metastasis | ||
| N0 | 1.71, 1.47-1.99, <0.001 | 1.60, 1.34-1.90, <0.001 |
| N1-N3 | 1.06, 1.03-1.09, <0.001 | 1.06, 1.03-1.10, 0.001 |
| Distant metastasis | ||
| Positive | 1.08, 0.97-1.20, 0.140 | 1.07, 0.96-1.20, 0.193 |
| Negative | 1.08, 1.05-1.12, <0.001 | 1.08, 1.04-1.12, <0.001 |
| TNM stage | ||
| I-II | 1.50, 1.24-1.81, <0.001 | 1.36, 1.08-1.70, 0.009 |
| III-IV | 1.06, 1.03-1.09, <0.001 | 1.06, 1.03-1.10, <0.001 |
| The Lauren's classification | ||
| Diffuse type | 1.19, 1.14-1.25, <0.001 | 1.18, 1.12-1.25, <0.001 |
| Intestinal type | 1.06, 1.01-1.11. 0.011 | 1.06, 1.01-1.12, 0.026 |
| Embolus | ||
| Positive | 1.20, 1.13-1.28, <0.001 | 1.19, 1.11-1.28, <0.001 |
| Negative | 1.08, 1.04-1.12, <0.001 | 1.08, 1.04-1.12, <0.001 |
| Tumor size | ||
| ≤ 4.5 cm | 1.21, 1.12-1.30, <0.001 | 1.17, 1.07-1.28, <0.001 |
| > 4.5 cm | 1.05, 1.02-1.09, 0.003 | 1.06, 1.02-1.10, 0.002 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval. The effect-size estimates were calculated under the Weibull proportional hazards regression models. *P was adjusted for age, gender, body mass index, smoking, drinking and family cancer history.
Some limitations should be considered when interpreting the results. First, our findings of this prospective cohort study can only be applied to the type of population enrolled in this study, i.e., postoperative patients for gastric cancer. Second, the maximal follow-up period in this study was 15 years and the minimal period was 5 years, and the recruitment phase lasted across 10 years, while might introduce a possible selection bias. Third, data on Helicobacter pylori infection are not available for us, and Helicobacter pylori infection has been proven to play an important role in gastric carcinogenesis. In addition, data on patients' nutritional conditions are also not available for us. So the residual confounding roles of Helicobacter pylori infection and nutritional status in the prognosis of preoperative NLR for gastric cancer mortality cannot be excluded. Fourth, all study patients were consecutively enrolled from a mono-center (Fujian Provincial Cancer Hospital) between early 2000 and late 2010, and during this 10-year period remarkable advances in surgical techniques might introduce a possible bias, which might underestimate the impact of blood-routine parameters on gastric cancer mortality.
Taken together, our findings not only consolidated the prognostic value of preoperative NLR and demonstrated that elevated NLR can predict poor survival in patients with early stage gastric cancer receiving radical gastrectomy, but also optimized the discrimination accuracy of NLR by recommending cutoff values in patients with TNM stage I and II. However, we agree that these cutoff values are tentative and may form the basis of a well-designed prospective study to test their validity. For practical reasons, our findings may provide the basis for future personalized medicine whereby gastric cancer patients with altered preoperative blood-routine parameters who are prone to experience a poor survival experience can be detected early and receive targeted therapeutic interventions.
Acknowledgements
We thank our colleagues over the years at the Fujian Provincial Cancer Hospital — particularly Xiaohui Chen, Yuzhen Zheng, Qingfeng Zheng, Shuoyan Liu, Zhilian She, Kunshou Zhu, Weidong Zang, Weizhong Ruan, Weimin Fang, Lin Li, Mingqiang Chen, Derong Zhang, Shaofeng Lin, Shunjin Chen, Yigui Chen and Guohong Zhao for performing the surgery, Yanni Gao, Zhenzhou Xiao, Su Lin, Chao Li, Xuehong Liao, Wenhui Jiang, Jieqiong Lin, Xinjing Li, Yan Xia, Yi Shi, Xiaojiang Wang, Shanfeng Jin, Hongfei Wang, Wucheng Shen, Weifeng Zhu, Xiaowen Cai, Baozhen Chen, Tongmei Chen, Xueyan Chen and Lifang Chen for collecting the blood/tissue samples and performing the follow-up investigations.
Funding
This study was financially supported by the National Natural Science Foundation of China (grant no. 81470111), the Natural Science Foundation of Fujian Province (grant nos. 2015J01451, 2016J01508), the Training Project for Young and Middle-Aged Core Talents of Health System of Fujian Province (grant nos. 2015-ZQN-JC-7, 2015-ZQN-JC-22), the National Clinical Key Specialty Construction Program (grant no. 2013-544), and the Ministry of Health P.R. China (grant no. WKJ2016-2-05).
Contributors
W.N., X.Z., F.P. drafted the protocol; D.H., H.Z., X.L., G.C., B.L., Y.C., Z.C. obtained statutory and ethics approvals; D.H., H.Z., X.L., C.L. B.L., G.C., Y.C., Z.C. contributed to data acquisition; D.H., F.P., X.Z., W.N. had access to all raw data; D.H., H.Z., W.N. did the data preparation, quality control and analyses, and checked the results; W.N., D.H. drafted the report. All authors contributed to writing the final report and approved the version to be published.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-13
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
3. Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-90
4. Gonzalez CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer. 2013;133:1023-32
5. Choi YY, Bae JM, An JY, Kwon IG, Cho I, Shin HB. et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014;110:129-35
6. Wang Y, Zhou LB, Li XH. S100A4 expression and prognosis of gastric cancer: a meta-analysis. Genet Mol Res. 2014;13:10398-403
7. Huang JY, Xu YY, Li M, Sun Z, Zhu Z, Song YX. et al. The prognostic impact of occult lymph node metastasis in node-negative gastric cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:3927-34
8. Alexiou D, Karayiannakis AJ, Syrigos KN, Zbar A, Sekara E, Michail P. et al. Clinical significance of serum levels of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in gastric cancer patients. Am J Gastroenterol. 2003;98:478-85
9. Pereira TV, Patsopoulos NA, Pereira AC, Krieger JE. Strategies for genetic model specification in the screening of genome-wide meta-analysis signals for further replication. Int J Epidemiol. 2011;40:457-69
10. Han MA, Kim YW, Choi IJ, Oh MG, Kim CG, Lee JY. et al. Association of smoking history with cancer recurrence and survival in stage III-IV male gastric cancer patients. Cancer Epidemiol Biomarkers Prev. 2013;22:1805-12
11. Peleteiro B, Lopes C, Figueiredo C, Lunet N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104:198-207
12. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut. 2010;59:39-48
13. Ock CY, Nam AR, Lee J, Bang JH, Lee KH, Han SW. et al. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer. 2016
14. Mohri Y, Tanaka K, Toiyama Y, Ohi M, Yasuda H, Inoue Y. et al. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine (Baltimore). 2016;95:e3125
15. Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D. et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol. 2016;27:687-92
16. Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH. et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350
17. Sun J, Chen X, Gao P, Song Y, Huang X, Yang Y. et al. Can the Neutrophil to Lymphocyte Ratio Be Used to Determine Gastric Cancer Treatment Outcomes? A Systematic Review and Meta-Analysis. Dis Markers. 2016;2016:7862469
18. Aldemir MN, Turkeli M, Simsek M, Yildirim N, Bilen Y, Yetimoglu H. et al. Prognostic Value of Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios in Local and Advanced Gastric Cancer Patients. Asian Pac J Cancer Prev. 2015;16:5933-7
19. Pang W, Lou N, Jin C, Hu C, Arvine C, Zhu G. et al. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol. 2016;28:493-502
20. Hu D, Peng F, Lin X, Chen G, Zhang H, Liang B. et al. Preoperative Metabolic Syndrome Is Predictive of Significant Gastric Cancer Mortality after Gastrectomy: The Fujian Prospective Investigation of Cancer (FIESTA) Study. EBioMedicine. 2017;15:73-80
21. Hu D, Lin X, Chen Y, Chang Q, Chen G, Li C. et al. Preoperative blood-routine markers and prognosis of esophageal squamous cell carcinoma: The Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. 2016
22. Peng F, Hu D, Lin X, Chen G, Liang B, Zhang H. et al. Preoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: The Fujian prospective investigation of cancer (FIESTA) study. Int J Cancer. 2016;139:2705-13
23. Hu D, Peng F, Lin X, Chen G, Liang B, Li C. et al. The elevated preoperative fasting blood glucose predicts a poor prognosis in patients with esophageal squamous cell carcinoma: The Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. 2016;7:65247-56
24. Peng F, Hu D, Lin X, Chen G, Liang B, Li C. et al. The monocyte to red blood cell count ratio is a strong predictor of postoperative survival in colorectal cancer patients: The Fujian prospective investigation of cancer (FIESTA) study. Journal of Cancer. 2017 December 24 [Accepted for publication]
25. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-4
26. Mohri Y, Tanaka K, Ohi M, Saigusa S, Yasuda H, Toiyama Y. et al. Identification of prognostic factors and surgical indications for metastatic gastric cancer. BMC Cancer. 2014;14:409
27. Aurello P, Tierno SM, Berardi G, Tomassini F, Magistri P, D'Angelo F. et al. Value of preoperative inflammation-based prognostic scores in predicting overall survival and disease-free survival in patients with gastric cancer. Ann Surg Oncol. 2014;21:1998-2004
28. Kim IY, You SH, Kim YW. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014;14:94
29. Bremnes RM, Busund LT, Kilvaer TL, Andersen S, Richardsen E, Paulsen EE. et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J Thorac Oncol. 2016
30. Cesana GC, Romano F, Piacentini G, Scotti M, Brenna A, Bovo G. et al. Low-dose interleukin-2 administered pre-operatively to patients with gastric cancer activates peripheral and peritumoral lymphocytes but does not affect prognosis. Ann Surg Oncol. 2007;14:1295-304
31. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-7
32. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584-96
33. Cho WC, Kwan CK, Yau S, So PP, Poon PC, Au JS. The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets. 2011;15:1127-37
34. Menges T, Engel J, Welters I, Wagner RM, Little S, Ruwoldt R. et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27:733-40
Author contact
![]() Corresponding authors: Wenquan Niu, Ph.D., Xiongwei Zheng, M.D. Ph.D. and Feng Peng, M.D. Ph.D. Address: Rui Jin Second Road 197, Huang Pu District, Shanghai 200025, China. Phone: +86-21-64370045 ext. 610905; Fax: +86-21-64333548; E-mail: niuwenquan_shcncom or nwq11588com.cn (W.N.) or agu1960com (X.Z.) or pengfengcom.cn (F.P.).
Corresponding authors: Wenquan Niu, Ph.D., Xiongwei Zheng, M.D. Ph.D. and Feng Peng, M.D. Ph.D. Address: Rui Jin Second Road 197, Huang Pu District, Shanghai 200025, China. Phone: +86-21-64370045 ext. 610905; Fax: +86-21-64333548; E-mail: niuwenquan_shcncom or nwq11588com.cn (W.N.) or agu1960com (X.Z.) or pengfengcom.cn (F.P.).

 Global reach, higher impact
Global reach, higher impact