3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(5):825-831. doi:10.7150/jca.17663 This issue Cite
Research Paper
Class I versus Class III radical hysterectomy in stage IB1 (tumor ≤ 2 cm) cervical cancer: a matched cohort study
Department of Obstetrics and Gynecology, the First Affiliated Hospital, Sun Yat-sen University, Zhongshan Second Road 58, Guangzhou 510700, China.
* These authors contributed equally to this article.
Received 2016-9-21; Accepted 2016-11-29; Published 2017-2-25
Abstract
Background & Aims: The long-term oncological outcome of Class I hysterectomy to treat stage IB1 cervical cancer is unclear. The aim of the present study was to compare the surgical and long-term oncological outcomes of Class I hysterectomy and Class III radical hysterectomy for treatment of stage IB1 cervical cancer (tumor ≤ 2 cm).
Methods: Seventy stage IB1 cervical cancer patients (tumor ≤ 2 cm) underwent Class I hysterectomy and 577 stage IB1 cervical cancer patients (tumor ≤ 2 cm) underwent Class III radical hysterectomy were matched with known risk factors for recurrence by greedy algorithm. Clinical, pathologic and follow-up data were retrospectively collected. Five-year survival outcomes were assessed using Kaplan-Meier model.
Results: After matching, a total of 70 patient pairs (Class I - Class III) were included. The median follow-up times were 75 (range, 26-170) months in the Class III group and 75 (range, 27-168) months in the Class I group. The Class I and Class III group had similar 5-year recurrence-free survival rates (RFS) (98.6% vs. 97.1%, P = 0.56) and overall survival rates (OS) (100.0% vs. 98.5%, P = 0.32). Compared with the Class III group, the Class I group resulted in significantly shorter operating time, less intra-operative blood loss, less intraoperative complications, less postoperative complications, and shorter hospital stay.
Conclusions: These findings suggest that Class I hysterectomy is an oncological safe alternative to Class III radical hysterectomy in treatment of stage IB1 cervical cancer (tumor ≤ 2 cm) and Class I hysterectomy is associated with fewer perioperative complication and earlier recovery.
Keywords: Cervical cancer, Oncological outcomes, Surgical outcomes, Class I hysterectomy, Class III radical hysterectomy.
Introduction
Cervical cancer is the second most common cancer and third leading cause of cancer death among females in less developed countries [1]. There are an estimated 98, 900 new cases and 30, 500 related deaths annually in China [2]. The standard surgical modality for women diagnosed with FIGO (International Federation of Gynecology and Obstetrics) stage IB1 cervical cancer is Class III radical hysterectomy (according to the Piver classification) and pelvic lymphadenectomy. For patients of stage IB1 cervical cancer with tumor ≤ 2 cm who want to preserve fertility, radical trachelectomy and pelvic lymphadenectomy is an alternative surgical treatment [3-5]. However, both radical hysterectomy and radical trachelectomy are correlated with significant surgical morbidity, such as blood loss, bladder dysfunction, sexual dysfunction, colorectal motility disorders, and fistula formation. The majority of these complications resulted from the removal of parametrium [6, 7].
Several studies have shown very low rates (less than 1%) of parametrial involvement in stage IB1 cervical cancer with tumor ≤ 2 cm [6, 8-13]. These data have raised the possibility that more conservative surgical modality may be appropriate for patients of stage IB1 cervical cancer with tumor ≤ 2 cm. Several retrospective studies have reported initial data suggesting the feasibility and safety of Class I hysterectomy in patients of stage IB1 cervical cancer with tumor ≤ 2 cm [6, 14-18]. However, before Class I hysterectomy as standard surgical alternative for stage IB1 cervical cancer with tumor ≤ 2 cm, we should compare the surgical outcomes and oncological outcomes with conventional golden standard (Class III radical hysterectomy). Although three prospective trials are investigating less radical surgery in patients with low-risk early-stage cervical cancer [12], the long-term oncological outcome of Class I hysterectomy to treat stage IB1 cervical cancer with tumor ≤ 2 cm is still unclear. The aim of the present study was to investigate the long-term oncological outcomes and surgical outcomes of Class I hysterectomy plus pelvic lymphadenectomy and Class III radical hysterectomy plus pelvic lymphadenectomy for treatment of stage IB1 cervical cancer with tumor ≤ 2 cm by matching risk factors for recurrence, using our 14-year, large scale database.
Patients and Methods
Patients
The study was approved by the Ethical Committee of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Informed consent was obtained from each patient. Class I hysterectomy plus pelvic lymphadenectomy via laparoscopy was recommended to patients of stage IB1 cervical cancer with tumor ≤ 2 cm as an alternative to Class III radical hysterectomy plus pelvic lymphadenectomy in our department from May 2002. The inclusion criteria of patients suitable of Class I hysterectomy were International Federation of Gynecology and Obstetrics (FIGO) stage IB1; tumor size ≤ 2 cm; no desire for fertility; stromal invasion ≤ 10 mm on loop electrosurgical excision procedure (LEEP) /conization biopsy; squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma; no evidence of lymph node metastasis before and during surgery; no lymphovascular space invasion (LVSI).
A second review of the histopathology was performed in all cases if the biopsy was done out of our hospital. Stromal invasion, LVSI, histotype, and grading of tumor were assessed by LEEP/conization biopsy. Tumor size was measured by the means of colposcopy and magnetic resonance imaging (MRI). Lymph node status was assessed by MRI or computer tomography (CT) scan before surgery. Gynecologic examinations by two experienced gynaecological oncologist were performed for staging purposes.
All suitable patients were determined after discussion in the multidisciplinary team meeting with the presence of pathologists specialized in gynaecological malignancies and gynaecological oncologists. Following the multidisciplinary team meeting a senior gynaecological oncologist assessed all patients and explained the proposed method of less radical surgery to all patients. It was emphasized that Class I hysterectomy plus pelvic lymphadenectomy was not the standard of surgical treatment for cervical cancer. The advantage of a less radical approach in terms of reduced morbidity was explained and consent was obtained.
A retrospective review of stage IB1 cervical cancer patients who underwent Class I hysterectomy plus pelvic lymphadenectomy or Class III radical hysterectomy plus pelvic lymphadenectomy at the First Affiliated Hospital of Sun Yat-sen University between 2002 and 2014 was done. Inclusion criteria of the Class III group were the same as the Class I group.
Study Design
To reduce the effects of potential confounding and selection bias, individual patient matching was done to make the risk factors for recurrence equal between the two groups. We matched cervical cancer patients with known intermediate and high risk factors for recurrence between the Class I and the Class III groups. Intermediate risk factors for recurrence include: deep stromal invasion, LVSI, and tumor size > 4 cm. High risk factors for recurrence include: parametrial involvement, resection margin involvement, and lymph node metastasis [3, 19, 20].
Surgical procedure
During the counseling period, we clearly introduced the advantages and disadvantages of each therapeutic surgery modality. Informed consent was obtained from each patient before surgery. The Class I hysterectomy plus pelvic lymphadenectomy via laparoscopy for stage IB1 cervical cancer patients were performed by a single, expert gynaecological surgical team from 2002. To enhance the objectivity of the comparison, the selection of the Class III group was limited to the patients who were operated by the same surgical team in the same period. In our center, patients with stage IB1 cervical cancer with tumor ≤ 2 cm conventionally underwent Class III radical hysterectomy plus lymphadenectomy via laparoscopy, according to the Piver classification [21]. Pelvic lymph node dissection (PLND) was performed in all patients. Para-aortic lymph node dissection (PALND) was not performed in the two groups.
Class I radical hysterectomy include extrafascial hysterectomy and removal of upper third of the vagina. The main surgical steps of Class III radical hysterectomy are: en bloc dissection of the uterus with the upper third of the vagina with the paravaginal and paracervical tissues. The uterine artery is ligated at their origin from internal iliac artery. The pararectal space and paravesicle space are opened to the pelvic floor. The parametria is resected bilaterally to the pelvic wall. The surgical specimens comprise the entire cervix, uterus, bilateral parametria, and vagina cuff. Bilateral pelvic lymphadenectomy include the removal of all nodal tissue from the external, internal, and common iliac vessels. The obturator nodes are also dissected from the obturator fossa including the lymph nodes collected below the obturator nerve to the pelvic floor. The distal margin of the pelvic nodal dissection is the deep circumflex iliac vein with the proximal limit of the dissection being the aortic bifurcation [21].
Postoperative adjuvant therapy and follow-up
After operation, cervical cancer patients with one or more high risk factors (parametrial involvement, lymph node metastasis, and resection margin involvement) were recommended to receive adjuvant concurrent chemoradiation therapy (CCRT). Patients with two or more intermediate risk factors (deep stromal invasion, LVSI, and tumor > 4 cm) were recommended to receive adjuvant radiation therapy [3, 22, 23].
After treatment completion, patients were followed up every 3 months in the first 2 years, every 6 months in the next 3 years and annually thereafter [24].
Definition
Blood loss was calculated as the sum of suctioned fluids and weighed gauze at completion of surgery. Operative duration was calculated from the time of skin incision to the closure of the skin. Complications were defined as any event during and after surgery that required further management. Bladder dysfunction was defined as the residual urine more than 100 ml over 14 days after surgery. Hospital stay was counted from the first postoperative day. Overall survival (OS) time was measured from the date of surgery to the date of death or censoring. Recurrence-free survival (RFS) time was calculated from the date of surgery to the date of recurrence or censoring.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA), SAS software (version 9.1; SAS institute Inc., Cary, NC), and MedCalc statistical software (MedCalc Software, Mariakerke, Belgium). To obtain pairs of subjects, we randomly selected a case patient from the Class I group and matched it to a control patient in the Class III group using a greedy algorithm as previous study [25]. The cases (Class I group) were ordered and sequentially matched to the nearest unmatched control (Class III group) according to matching criteria. Variables were compared between the two groups by paired t-test or Wilcoxon signed rank test for continuous data and McNemar's test or marginal homogeneity test for categorical data. Survival curves were created by the Kaplan-Meier method. Differences between survival curves were calculated by the log-rank test. P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
A total of 70 patients who underwent Class I hysterectomy plus lymphadenectomy and 577 patients underwent Class III radical hysterectomy plus lymphadenectomy were eligible for this study. After matching, each group (Class I and Class III) contained 70 cervical cancer patients (Fig. 1). The characteristics of patients and tumors of the two groups are summarized in Table 1. There were no significant differences in age, parity, previous surgery, FIGO stage, tumor differentiation, and histological type between the two groups (P > 0.05). Mean body mass index (23.83 kg/m2 vs. 21.69 kg/m2) were significantly higher in the Class III group than in the Class I group (P = 0). There were no differences in risk factors for recurrence between the Class I and Class III groups (P = 1).
Surgical data
The mean operating time (226.43 min vs. 131.21 min, P = 0), estimated blood loss (224.29 ml vs. 100.57 ml, P = 0), blood transfusion (7.14% vs. 0, P = 0.03), the duration of bowel motility return (2.31 days vs. 1.34 days, P = 0) and hospital stay (9.30 days vs. 2.96 days, P = 0) were significantly higher in the Class III group than in the Class I group. There were no significant differences in the number of pelvic lymph node removed (30.87 vs. 28.69, P = 0.61) between the two groups (Table 2).
Study design.
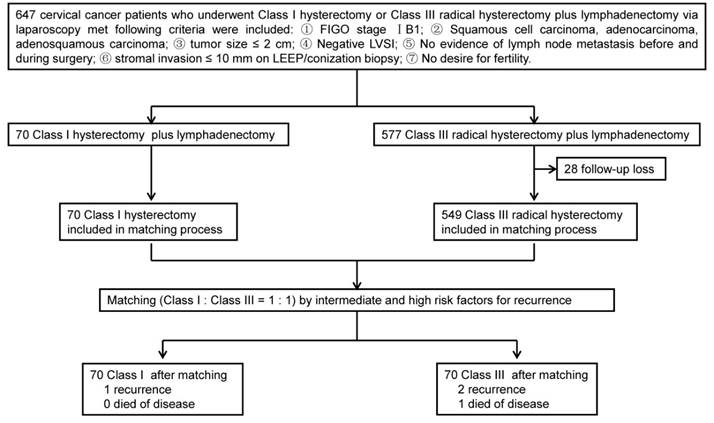
Clinicopathologic characteristics of patients.
| Characteristics | Class III group (n = 70) | Class I group (n = 70) | P |
|---|---|---|---|
| Age (years) (mean±SD) | 43.03 ± 8.59 | 44.04 ± 8.46 | 0.44 |
| Parity (mean±SD) | 1.45 ± 0.88 | 1.58 ± 0.99 | 0.30 |
| BMI (Kg/m2) (mean±SD) | 23.83 ± 3.57 | 21.69 ± 3.91 | 0 |
| Previous surgery, n (%) | 24 (34.29) | 23 (32.86) | 0.86 |
| FIGO stage, n (%) | 1 | ||
| ⅠB1 | 70 (100.00) | 70 (100.00) | |
| Histological type, n (%) | 0.71 | ||
| Squamous | 58 (82.86) | 53 (75.71) | |
| Adenocarcinoma | 9 (12.86) | 17 (24.29) | |
| Adenosquamous | 3 (4.28) | 0 (0) | |
| Grade, n (%) | 0.09 | ||
| G1 | 18 (25.71) | 9 (12.86) | |
| G2 | 24 (34.29) | 27 (38.57) | |
| G3 | 28 (40.00) | 34 (48.57) | |
| Tumor size (cm) n (%) | 1 | ||
| ≤ 4 | 70 (100) | 70 (100) | |
| > 4 | 0 | 0 | |
| Stromal invasion, n (%) | 1 | ||
| < 1/2 | 70 (100) | 70 (100) | |
| ≥ 1/2 | 0 | 0 | |
| LVSI, n (%) | 1 | ||
| Positive | 0 | 0 | |
| Negative | 70 (100) | 70 (100) | |
| Parametrium involvement, n (%) | 1 | ||
| Positive | 0 | 0 | |
| Negative | 70 (100) | 70 (100) | |
| LNM, n (%) | 1 | ||
| Positive | 2 (2.86) | 2 (2.86) | |
| Negative | 68 (97.14) | 68 (97.14) | |
| Margin involvement, n (%) | 1 | ||
| Positive | 0 | 0 | |
| Negative | 70 (100) | 70 (100) |
Class I, the Class I hysterectomy according to the Piver classification; Class III, the Class III hysterectomy according to the Piver classification; BMI, body mass index; FIGO, international Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; LNM, lymph node metastasis.
Operative details and surgical outcomes
| Class III group (n = 70) | Class I group (n = 70) | P | |
|---|---|---|---|
| Operating time (min) (mean±SD) | 226.43 ± 44.21 | 131.21 ± 37.88 | 0 |
| Blood loss (ml) (mean±SD) | 224.29 ± 124.45 | 100.57 ± 46.59 | 0 |
| Blood transfusion, n (%) | 5 (7.14) | 0 | 0.03 |
| Pelvic lymph node retrieval (mean±SD) | 30.87 ± 8.46 | 28.69 ± 5.87 | 0.61 |
| Return of bowel movement (days) (mean±SD) | 2.31 ± 1.23 | 1.34 ± 0.54 | 0 |
| Hospital stay (days) (mean±SD) | 9.30 ± 4.99 | 2.96 ± 0.77 | 0 |
Class I, the Class I hysterectomy according to the Piver classification; Class III, the Class III hysterectomy according to the Piver classification.
Intraoperative and postoperative complications
The rate of intraoperative complications was significantly higher in the Class III group than in the Class I group (P = 0.014). In the Class III group, intraoperative complications occurred in 6 patients, including Major vessel injury (1), ureter injury (1), bladder injury (3), and hypercapnia (1). The major vessel injury was right common iliac vein laceration. All the intraoperative complications were handled under laparoscopy. No intraoperative complications were recorded in the Class I group. The rate of postoperative complications was significantly higher in the Class III group than in the Class I group (P = 0.035). Rates of bladder dysfunction (74.29% vs. 0, P = 0) was significantly higher in the Class III group than in the Class I group (Table 3).
Intraoperative and postoperative complications
| Class III group (n = 70) | Class I group (n = 70) | P | |
|---|---|---|---|
| Intraoperative complications, n (%) | 0.014 | ||
| Bladder injury | 3 (4.29) | 0 | 0.08 |
| Major vessel injury | 1 (1.43) | 0 | 0.32 |
| Ureter injury | 1 (1.43) | 0 | 0.32 |
| Hypercapnia | 1 (1.43) | 0 | 0.32 |
| Postoperative complication excluding bladder dysfunction, n (%) | 0.035 | ||
| Vaginal stump bleeding | 1 (1.43) | 0 | 0.32 |
| Ileus | 2 (2.86) | 0 | 0.16 |
| Vesicovaginal fistula | 2 (2.86) | 0 | 0.16 |
| Symptomatic lymphocyst | 2 (2.86) | 2 (2.86) | 1 |
| Lymphedema | 1 (1.43) | 1 (1.43) | 1 |
| Ureterostenosis | 1 (1.43) | 0 | 0.32 |
| Deep vein thrombosis | 1 (1.43) | 0 | 0.32 |
| Bladder dysfunction | 52 (74.29) | 0 | 0 |
Class I, the Class I hysterectomy according to the Piver classification; Class III, the Class III hysterectomy according to the Piver classification.
Oncological date
After surgery, 2 (2.86%) patients in the Class III group and 2 (2.86%) patients in the Class I group received adjuvant therapy after surgery according to risk factors for recurrence. There were no significant differences in the type of adjuvant therapy between the two groups (P = 1) (Table 4). The median follow-up times were 75 (range, 26-170) months in the Class III group and 75 (range, 27-168) months in the Class I group. During the follow-up, 2 recurrent patients in the Class III group and 1 recurrent patient in the Class I group were recorded. At the time of analysis, 1 patient in the Class III group died of disease and no patient in the Class I group died of disease (Table 5).
Survival outcomes did not significantly differ between the two groups by the Kaplan-Meier method. The 5-year RFS were 98.6% in the Class I group and 97.1% in the Class III group (P = 0.56) (Fig. 2a). The 5-year OS were 100.0% in the Class I group and 98.5% in the Class III group (P = 0.32) (Fig. 2b).
Kaplan-Meier analysis of oncological outcomes in patients with stage IB1 cervical cancer (tumor ≤ 2 cm) who underwent Class I or Class III radical hysterectomy. A Recurrence-free survival. B overall survival.
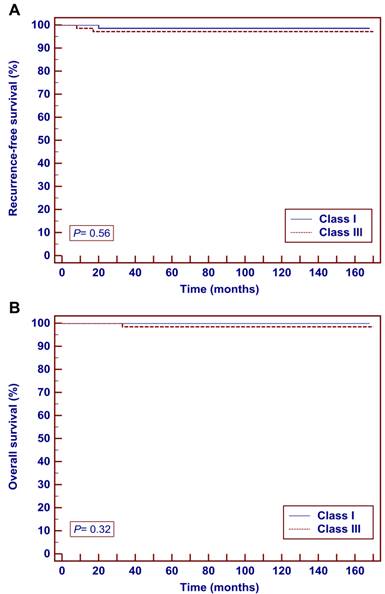
Survival outcomes.
| Class III group (n = 70) | Class I group (n = 70) | P | |
|---|---|---|---|
| Adjuvant therapy | 1 | ||
| No | 68 (97.14%) | 68 (97.14%) | 1 |
| RT | 0 | 0 | 1 |
| CCRT | 2 (2.86%) | 2 (2.86%) | 1 |
| Number of recurrence | 2 (2.86%) | 1 (1.43%) | 0.56 |
| Number of death due to recurrence | 1 (1.43%) | 0 | 0.32 |
Class I, the Class I hysterectomy according to the Piver classification; Class III, the Class III hysterectomy according to the Piver classification.
Characteristics of patients with disease recurrence
| No. | FIGO stage | Histology | Grade | Stromal invasion | LVSI | Tumor size | LNM | PM | RM | Site of metastasis | DFI (m) | Treatment | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| III | |||||||||||||
| 1 | Ⅰb1 | Adeno | G1 | < 1/2 | - | 0.5 | - | - | - | Pelvic cavity | 8 | CCRT | NED |
| 2 | Ib1 | Scc | G1 | < 1/2 | - | 1.5 | + | - | - | PALN | 17 | OP+CCRT | Expired |
| I | |||||||||||||
| 1 | Ⅰb1 | Scc | G3 | < 1/2 | - | 0.8 | - | - | - | Pelvic cavity | 20 | CCRT | NED |
I, the Class I hysterectomy according to the Piver classification; III, the Class III hysterectomy according to the Piver classification; LVSI, lymphovascular space invasion; LNM, lymph node metastasis; PM, parametrial metastasis; RM, resection margin metastasis; DFI, disease free interval; OP, operation; CCRT, concurrent chemoradiation therapy; NED, no evidence of disease; SCC, squamous cell carcinoma; Adeno, adenocarcinoma; FIGO, international Federation of Gynecology and Obstetrics.
Discussion
In this study, with matching known intermediate and high risk factors for recurrence, we found similar oncological outcomes between Class I hysterectomy and Class III radical hysterectomy for patients of stage IB1 cervical cancer with tumor size ≤ 2 cm. Moreover, compared with the Class III group, the Class I group had more operative advantages including shorter operation time, less intra-operative blood loss, lower transfusion rate, faster recovery, fewer perioperative complications, and shorter hospital stay.
Class III radical hysterectomy plus lymphadenectomy were deemed as the standard surgical treatment for stage IB1 cervical cancer patients [3, 26, 27]. Although the oncological outcomes of patients with stage IB1 cervical cancer treated by Class III radical hysterectomy is excellent, patients frequently suffer from lower urinary tract dysfunction, colorectal motility disorders, and sexual dysfunction associated with autonomic nerve damage resulted from wide resection of the parametrium [6, 7]. In recent years, more concern has been focused on the question of identifying low-risk criteria for parametrial involvement in early stage cervical cancer to avoid radical resection of parametrium. Several retrospective studies have shown very low rates of parametrial involvement (approximately 1% or less) in patients with favorable pathologic characteristics (tumor ≤ 2 cm, stromal invasion ≤ 1/2, absence of LVSI, and negative lymph nodes metastasis on frozen section) [6, 8-13]. In this study, we found no parametrial involvement in stage IB1 cervical cancer patients with tumor ≤ 2 cm, which is similar to previous study. AI-Kalbani et al. retrospectively reviewed 20 patients of stage IB1 cervical cancer (tumor ≤ 2 cm) who underwent radical hysterectomy found no parametrial involvement [28]. Hirai et al. retrospectively observed 19 patients of stage IB1 cervical cancer (tumor ≤ 2 cm) who underwent radical hysterectomy found no parametrial involvement (0/19) [29]. These initial data suggest the feasibility and safety of Class I hysterectomy for stage IB1 cervical cancer patients with favorable pathologic characteristics.
To effectively compare the oncological outcomes, we matched stage IB1 patients from our database with the known intermediate and high risk factors for recurrence between the two groups (Class I and Class III). After matching, we enrolled 70 stage IB1 cervical cancer patients (tumor ≤ 2 cm) in each group with median follow-up time 75 months. In our study, the Class I and Class III groups had similar 5-year RFS (98.6% vs. 97.1%, P = 0.56) and OS rates (100.0% vs. 98.5%, P = 0.32). Our results are confirmed by previous studies. Biliatis et al. in a retrospective study of 27 small volume stage IB1 cervical cancer patients undergoing simple hysterectomy with median follow-up of 56 months found no patients died of disease [30]. Naik et al. retrospectively reviewed 12 small-volume stage IB1 cervcial cancer underwent simple hysterectomy with a mean follow-up of 29 months and found no recurrence [17]. However, these studies were single-arm researches and lacked control group. In addition, most of studies on less radical surgery reported outcomes of heterogonous stages of cervical cancer patients (including stage IA1, IA2, IB1, and IIA) and different surgical modalities (including cone biopsy, simple trachelectomy, simple hysterectomy, Class II radical hysterectomy with or without lymphadenectomy). Londoni et al. performed a prospective study to compare Class III radical hysterectomy (n = 63) and Class I hysterectomy (n = 62) for stage IB1 and IIA cervical cancer patients with tumor ≤ 4 cm [18]. They found no significant differences in RFS and OS between the two groups, which were similar to the present study. However, the 5-year OS were 85% and 95% respectively in the Class I and Class III group, which were lower than our data. This may be caused by the different cohort of patients with different inclusion criteria. In the present study, a total of 70 stage IB1 cervical cancer (tumor ≤ 2 cm) patient pairs (Class I - Class III) were included after matching with known risk factors for cervical cancer recurrence. We found similar 5-year recurrence-free survival rates (RFS) (98.6% vs. 97.1%, P = 0.56) and overall survival rates (OS) (100.0% vs. 98.5%, P = 0.32) between the Class I and Class III group. Therefore, to our knowledge, the present study is the first evaluating outcomes between two homogenized comparable groups for exclusive stage IB1 cervical cancer patients with tumor ≤ 2 cm. This represents the main strength of the present study.
Due to the success of cervical screening programs in recent years, many more patients of cervical cancer are diagnosed at an earlier stage. Most of these women request that they are given choices in the management of their disease and show a more concern on the perioperative complications. In the present study, the rates of complications related to surgery were significantly higher in the Class III group than in the Class I group. Previous studies showed that the incidence of postoperative bladder dysfunction after radical hysterectomy ranged from 1.7% to 76% [31, 32]. Bladder dysfunctions after surgery were exclusive to Class III radical hysterectomy (74.29%) in the present study. These data support that the perioperative morbidity (especially urologic) is proportional to the extent of the parametrial resection.
Our study is a single-center retrospective research, which may contain selection and confounding bias. To overcome the limitation, we matched patients in the two groups with known intermediate and high risk factors of recurrence, which can minimize the impact of potential bias on the oncological outcomes. Since the non-randomized nature of the study, we emphasized that our results are preliminary and further prospective study are necessary.
In conclusion, our results showed that Class I hysterectomy plus lymphadenectomy is an oncological safe surgical treatment with less perioperative complications for patients of stage IB1 cervical cancer with tumor ≤ 2 cm, compared with Class III radical hysterectomy. Although multicenter randomized controlled trials are needed, our investigation provides data basis to support Class I hysterectomy for the treatment of stage IB1 cervical cancer (tumor ≤ 2 cm).
Abbreviations
RFS: Recurrence-free survival rates; OS: Overall survival rates; FIGO: International Federation of Gynecology and Obstetrics; LVSI: Lymphovascular space invasion; MRI: Magnetic resonance imaging; CT: Computer tomography; PLND: Pelvic lymph node dissection; PALND: Para-aortic lymph node dissection; CCRT: Concurrent chemoradiation therapy; BMI: Body mass index; PM: Parametrial metastasis; RM: Resection margin metastasis; DFI: Disease free interval; NED: No evidence of disease; SCC: Squamous cell carcinoma; Adeno: Adenocarcinoma; LEEP: loop electrosurgical excision procedure.
Acknowledgements
We thank Xiao Gong for the statistical analysis. This work is supported by the Sun Yat-sen University Clinical Research 5010 Program (Grant number 2007010), the Natural Science Foundation of Guangdong Province, China (Grant number 2016A030310147, 2015A030313073), and the Science and Technology Program of Guangzhou, China (Grant number 201510010289).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
3. Koh WJ, Greer BE, Abu-Rustum NR. et al. Cervical Cancer, Version 2.2015. Journal of the National Comprehensive Cancer Network. 2015;13:395-404 quiz
4. Ramirez PT, Pareja R, Rendon GJ. et al. Management of low-risk early-stage cervical cancer: should conization, simple trachelectomy, or simple hysterectomy replace radical surgery as the new standard of care? Gynecologic oncology. 2014;132:254-9
5. Dargent D, Martin X, Sacchetoni A. et al. Laparoscopic vaginal radical trachelectomy: a treatment to preserve the fertility of cervical carcinoma patients. Cancer. 2000;88:1877-82
6. Reade CJ, Eiriksson LR, Covens A. Surgery for early stage cervical cancer: how radical should it be? Gynecologic oncology. 2013;131:222-30
7. Wit EM, Horenblas S. Urological complications after treatment of cervical cancer. Nature reviews Urology. 2014;11:110-7
8. Wright JD, Grigsby PW, Brooks R. et al. Utility of parametrectomy for early stage cervical cancer treated with radical hysterectomy. Cancer. 2007;110:1281-6
9. Covens A, Rosen B, Murphy J. et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecologic oncology. 2002;84:145-9
10. Frumovitz M, Sun CC, Schmeler KM. et al. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstetrics and gynecology. 2009;114:93-9
11. Kinney WK, Hodge DO, Egorshin EV. et al. Identification of a low-risk subset of patients with stage IB invasive squamous cancer of the cervix possibly suited to less radical surgical treatment. Gynecologic oncology. 1995;57:3-6
12. Ramirez PT, Pareja R, Rendón GJ. et al. Management of low-risk early-stage cervical cancer: Should conization, simple trachelectomy, or simple hysterectomy replace radical surgery as the new standard of care? Gynecologic oncology. 2014;132:254-9
13. Li XL, Liu XX, Cao GS. et al. Narrowing Resection of Parametrial Tissues Is Feasible in Low-Risk Cases of Stage IA2-IB1 Cervical Cancer. Journal of Cancer. 2016;7:1481-6
14. Palaia I, Musella A, Bellati F. et al. Simple extrafascial trachelectomy and pelvic bilateral lymphadenectomy in early stage cervical cancer. Gynecologic oncology. 2012;126:78-81
15. Pluta M, Rob L, Charvat M. et al. Less radical surgery than radical hysterectomy in early stage cervical cancer: a pilot study. Gynecologic oncology. 2009;113:181-4
16. Bouchard-Fortier G, Reade CJ, Covens A. Non-radical surgery for small early-stage cervical cancer. Is it time? Gynecologic oncology. 2014;132:624-7
17. Naik R, Cross P, Nayar A. et al. Conservative surgical management of small-volume stage IB1 cervical cancer. BJOG. 2007;114:958-63
18. Landoni F, Maneo A, Zapardiel I. et al. Class I versus class III radical hysterectomy in stage IB1-IIA cervical cancer. A prospective randomized study. European journal of surgical oncology. 2012;38:203-9
19. Sevin BU, Lu Y, Bloch DA. et al. Surgically defined prognostic parameters in patients with early cervical carcinoma. A multivariate survival tree analysis. Cancer. 1996;78:1438-46
20. Zreik TG, Chambers JT, Chambers SK. Parametrial involvement, regardless of nodal status: a poor prognostic factor for cervical cancer. Obstetrics and gynecology. 1996;87:741-6
21. Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstetrics and gynecology. 1974;44:265-72
22. Rotman M, Sedlis A, Piedmonte MR. et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. International journal of radiation oncology, biology, physics. 2006;65:169-76
23. Monk BJ, Wang J, Im S. et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecologic oncology. 2005;96:721-8
24. Salani R, Backes FJ, Fung MF. et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. American journal of obstetrics and gynecology. 2011;204:466-78
25. Nam JH, Park JY, Kim DY. et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Annals of oncology. 2012;23:903-11
26. Landoni F, Maneo A, Colombo A. et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535-40
27. Landoni F, Maneo A, Cormio G. et al. Class II versus class III radical hysterectomy in stage IB-IIA cervical cancer: a prospective randomized study. Gynecologic oncology. 2001;80:3-12
28. Al-Kalbani M, McVeigh G, Nagar H. et al. Do FIGO stage IA and small (</=2 cm) IB1 cervical adenocarcinomas have a good prognosis and warrant less radical surgery? International journal of gynecological cancer. 2012;22:291-5
29. Hirai Y, Takeshima N, Tate S. et al. Early invasive cervical adenocarcinoma: its potential for nodal metastasis or recurrence. BJOG. 2003;110:241-6
30. Biliatis I, Kucukmetin A, Patel A. et al. Small volume stage 1B1 cervical cancer: Is radical surgery still necessary? Gynecologic oncology. 2012;126:73-7
31. Benedetti-Panici P, Zullo MA, Plotti F. et al. Long-term bladder function in patients with locally advanced cervical carcinoma treated with neoadjuvant chemotherapy and type 3-4 radical hysterectomy. Cancer. 2004;100:2110-7
32. Ayhan A, Tuncer ZS, Yarali H. Complications of radical hysterectomy in women with early stage cervical cancer: clinical analysis of 270 cases. European journal of surgical oncology. 1991;17:492-4
Author contact
![]() Corresponding author: Shu-zhong Yao, Department of Obstetrics and Gynecology, the First Affiliated Hospital, Sun Yat-sen University, Zhongshan Second Road 58, Guangzhou 510700, China. Tel: +8602087332200-8342 Fax: +8602087332200-8330 E-mail: yaoshuzhsysu.edu.cn.
Corresponding author: Shu-zhong Yao, Department of Obstetrics and Gynecology, the First Affiliated Hospital, Sun Yat-sen University, Zhongshan Second Road 58, Guangzhou 510700, China. Tel: +8602087332200-8342 Fax: +8602087332200-8330 E-mail: yaoshuzhsysu.edu.cn.

 Global reach, higher impact
Global reach, higher impact