3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(5):801-808. doi:10.7150/jca.17640 This issue Cite
Research Paper
Protein Regulator of Cytokinesis PRC1 Confers Chemoresistance and Predicts an Unfavorable Postoperative Survival of Hepatocellular Carcinoma Patients
1. Department of Gastroenterology, Qingdao Municipal Hospital, Medical College, Qingdao University, Qingdao, Shandong, China;
2. Department of Gastroenterology, Rizhao People's Hospital, Rizhao, Shandong, China;
3. Department of Digestive Medicine, Rizhao Traditional Chinese Medicine Hospital, Wanghai Road, Rizhao, Shandong, China;
4. Department of Gynaecology and Obstetrics, Rizhao Maternal and Child Health Hospital, Rizhao, Shandong, China;
5. Endoscope Center, Rizhao Traditional Chinese Medicine Hospital, Wanghai Road, Rizhao, Shandong, China;
6. Department of Pathology, Rizhao People's Hospital, Rizhao, Shandong, China;
7. Department of Pediatric Surgery, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, China;
8. Department of Cardiovascular Surgery of the First Affiliated Hospital& Institute for Cardiovascular Science, Soochow University, Suzhou, Jiangsu, China.
*These authors contributed equally to this work.
Received 2016-9-20; Accepted 2016-11-14; Published 2017-2-25
Abstract
Background: PRC1, a microtubules(MTs)-associated protein, is essential in the mitosis and cell cycle regulation. It has been recently linked to chemoresistance and tumorigenesis. The current study sought to explore the role of PRC1 on chemoresistance and postoperative prognosis of hepatocellular carcinoma(HCC).
Methods: PRC1 was transfected into HCC cells to detect its effects of chemoresistance to 5-fluorouracil in vitro and in vivo. This study also investigated the impact of PRC1 on 5-FU-induced G2/M phase arrest and the potential molecular mechanism. Surgical specimens from HCC patients were examined immunohistochemically for PRC1 expression.
Results: Ectopic expression of PRC1 significantly increased the chemoresistance, promoted the tumor growth and abrogated 5-FU-induced G2/M phase arrest via p21/p27-pRBs pathway. In clinical specimens, high expression of PRC1(immunostaining score≥3) in HCC cells predicted an unfavorable postoperative survival of HCC patients(P=0.019), especially for whom received postoperative chemotherapy(P=0.002). In multivariate Cox analyses, high PRC1 expression significantly predicted an unfavorable postoperative prognosis, not dependent of TNM stage.
Conclusion: High PRC1 expression in HCC cells increased chemoresistance, attenuated 5-FU-induced apoptosis, abrogated 5-FU-induced G2/M phase arrest, and predicts an unfavorable survival, especially for the patients who received chemotherapy. PRC1 might be a novel prognostic and predictive marker and therapeutic target for HCC patients.
Keywords: PRC1, chemoresistance, postoperative, survival, hepatocellular carcinoma.
Introduction
Hepatocellular carcinoma(HCC) is one of most common malignancies and the second leading cause of cancer death worldwide, with estimated 745,500 deaths per annum[1, 2]. The prognosis of HCC is really poor and surgery in combination with adjuvant chemotherapy offers the possibility of long-term survival for HCC patients. However, a part of patients benefit little from chemotherapy and develop platinum/5-florouracil(5-FU)-resistant relapse[3]. To characterize this heterogeneity, it is necessary to develop effect predictive/prognostic markers to identify HCC patients who will get better prognosis, which may provide a guidance for individualized therapeutic intervention of HCC.
PRC1 is a microtubules(MTs)-associated protein (MAP) which are involved in cytokinesis and MTs organization[4, 5]. MTs are very important in cell cycle, trafficking and chemoresistance. Cytotoxic agents trigger the mitotic checkpoint through interfering with mitotic spindle dynamics, thus induce cell G2/M-phase arrest & apoptosis [6, 7]. MAPs promote microtubule assembly/stabilization, and correlate with increased chemoresistance of cytotoxic agents[8]. As a member of MAPs, PRC1 was found a higher expression in HCC tissues compared with matched adjacent-non-cancer liver, in recurrence HCC compared with primary HCC tissues[9]. Further qPCR validated the results from data mining of microarray[10]. Chen et al. also reported the oncogenic properties of PRC1 in HCC[10]. But the effect of PRC1 in chemoresistance as a MAP was unknown. Therefore we detected the PRC1 expression in formalin-fixed paraffin embedded (FFPE) specimens from 222 HCC patients(123 patients received postoperative chemotherapy). Stratification analysis showed that high expression (immunohistochemistry score≥3) of PRC1 in patients received chemotherapy was significantly associated with a favorable prognosis after curative surgery. However, this effect was not found in those without chemotherapy. To summarize, PRC1 may be a novel prognosis factor in predicting the benefit of HCC patients received from chemotherapy.
In the current study, we report that PRC1 expression confer chemoresistance and predict an unfavorable postoperative prognosis of HCC patients, especially for those who received 5-FU/platinum chemotherapy. These intriguing findings reveal the important role of PRC1 in chemoresistance and provide a predictive marker for HCC prognosis.
Materials and Methods
HCC patients and postoperative Follow-up
Consecutive HCC patients who received curative surgery at Department of Gastroenterology, Rizhao People's Hospital(Rizhao, China) from Sep 2003 to May 2009 were enrolled in our study. All the patients were pathologically confirmed and signed the informed consent. Follow-up was performed via in person interview or telephone calls according to the standard epidemiologic procedure twice a year. The final date of follow-up was Dec 12, 2015. A total of 222 patients with intact follow-up information and PRC1 immunohistochemistry data were included in survival analysis. Freshly frozen and FFPE tumor specimens and the matched adjacent non-cancer tissues from 48 patients were collected for comparative analysis of PRC1 expression. All the protocols conformed to the Declaration of Helsinki and were approved by ethics committee of the Rizhao people's hospital.
Adenovirus preparation and HCC xenograft models construction
Ad-PRC1, a replication-deficient adenovirus vector expressing PRC1, was constructed as described[11]. The pAd-Easy1 adenovirus system was purchased from Addgene (Cambridge, MA, USA). The recombinants were identified with Pac I prior to transfection. 2 weeks later, the viruses were collected and expanded for 3 cycles. The viruses were purified according to the manufacturer's instruction(Clontech, Mountain View, CA). Before transduction the viruses should be examined at different titers. The animal study protocol was approved by the ethics committee of the Rizhao people's hospital. Female pathogen-free nude mice (nu/nu, 4-6 weeks) were purchased from Laboratory Animal Center of Qingdao University (Qingdao, China). To establish Huh7 HCC xenograft models, 1×106 cells in 100μL DMEM were subcutaneously injected into the right flank of each mice. All mice were monitored for activity, body weight, and tumor growth. While the tumor volume reached ~50 mm3, the mice bearing xenografts were randomly divided into four groups: Ad-Vector, Ad-PRC1, Ad-Vector+5-FU, Ad-PRC1+5-FU (n=10/group). A total of 2×109 infectious particles (IP) of Ad-Vector or Ad-PRC1 were intratumoral injected every 3 days; 5-FU(5 mg/kg) was intraperitoneal (i.p.) injected weekly for about 4 weeks[12-14]. The control group only received the vehicle.
Flow cytometry for cell cycle analysis
Cells were digested by 0.25% trypsin (Beyotime, Jiangsu, China), and fixed overnight in 70% cold ethanol at 4˚C. Then the cells were stained by 1 mL 50 μg/mL PI solution(0.1% Triton X-100 + 0.1 mg/ml RNase) for 30 min(dark, 37℃). The cell cycle were analysised on Beckman-coulter Gallios flow cytometer(Beckman Coulter Inc., Brea, CA). Flowjo software (Tree Star Inc., Ashland, OR, USA) was applid to calculate the percentages of cells in G2/M phase.
Western Blot
Protein was extracted, quantified, and separated by12% SDS-PAGE. Then it was transferred to PVDF membranes (Millipore, Bedford, USA), incubated with antibodies for Cip/p21, Kip/p27 and pRBs (Santa Cruz, 1:1000). Finally, the membranes were immersed in ECL substrate solution (Biorad, USA) and detected using SyngeneTM gel imaging analysis system. β-actin was an internal control.
Immunohistochemistry
The PRC1 expression in primary HCC specimens were detected by immunohistochemistry. Rabbit monoclonal antibody against PRC1 (ab51248, 1:100, Abcam) was applied for immunostaining. The score was independently evaluated by 2 researchers (Y.W., S.X.). They were blind to the clinical features of patients. Immunostaining scores were calculated from the multiplication of the intensity and the extent. The intensity was graded as 0(-, negative), 1(+, weak), 2(++, moderate), and 3(+++, intense). The extent of positive staining cells was classified as 0(0-4%), 1(5-24%), 2(25-49%), 3(50-74%), 4(75-100%). After fully evaluated, a mean score of 3 random selected visual fields were given to each specimen. The median score of 3 was determined as the cutoff point best dichotomized patients into high-expression and low-expression groups. Quantitative analysis was performed with the Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, USA). The stained sections were examined at 200× magnification. 10 representative staining fields/each section were evaluated to generate the Mean Optical Density (MOD). MOD represents the intensity of staining signals as measured positive pixels. The negative control was used for background subtraction. The average MOD difference between two groups was statistically analyzed by t-test, and P<0.05 was considered significant.
Statistics
Chi-square test and Student t test were used to detect the differences in categorical variables and continual variables, respectively. The correlation between immunostaining score and clinical features was determined by Spearman rank test. The survival was evaluated by Kaplan-Meier analysis with log-rank test. Cox regression analysis was employed to generate hazard ratio and correlated 95% confidence interval of factors which were associated with HCC-specific survival. All statistical tests were 2-side and performed with SPSS 18.0 for windows (SPSS, Chicago, III). P<0.05 were considered significant.
Results
Ectopic Expression of PRC1 Increased Chemoresistance to 5-FU and Conferred Resistance to Cytotoxic Agent-Induced Apoptosis
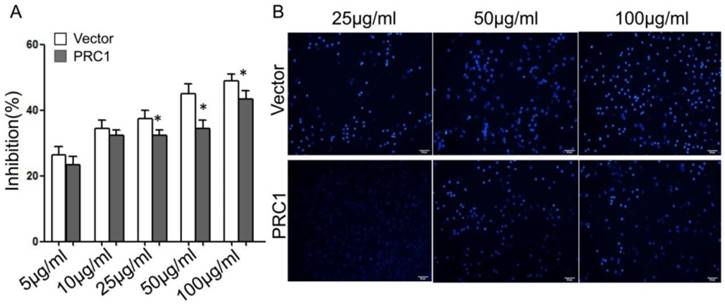
PRC1-transfected and empty-vector-transfected Huh-7 cells were tested by CCK-8 assay for their sensitivity to 5-FU. The IC50 concentrations were 192.3 μg/mL, 146.7μg/mL, respectively. Ectopic expression of PRC1 notably increase the chemoresistance of HCC cells to 5-FU (Fig. 1A). After being treated with 5-FU at different concentration, the ratio of apoptotic cells was calculated. The ratio in empty-vector-transfected Huh-7 cells was significantly higher than that in PRC1-transfected Huh-7 cells (25μg/mL: 15.7%±2.1% vs. 5.6%±1.3%, P=0.019; 50μg/mL: 25.9±3.4% vs. 14.5±2.6%, P=0.005; 100μg/mL: 43.7%±9.1% vs. 21.6%±5.2%, P=0.011) (Fig. 1B).
PRC1 ectopic expression increased Chemoresistance of HCC cells and conferred resistance to 5-FU-induced apoptosis. (A)CCK-8 assay was used to evaluate the cell growth inhibition of 5-FU in Vector or PRC1 groups; (B) The apoptosis status of cells from Vector or PRC1 groups was examined under a fluorescence microscope after Hoechst 33258 staining. All experiments were performed in triplicate. Data was presented as mean ±SD.
PRC1 promoted the growth of xenograft tumors and increased the resistance of tumors to 5-FU in vivo
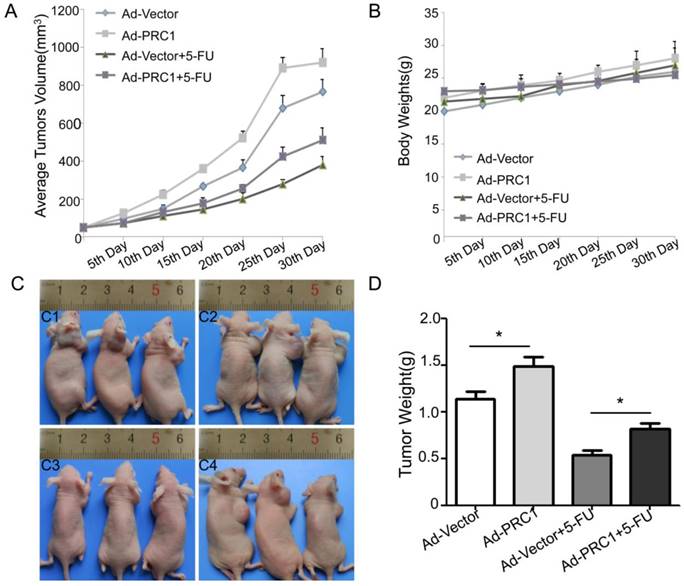
To detect the chemoresistance effect of PRC1 in vivo, nude mice bearing HCC xenograft tumors were intratumorally injected with Ad-PRC1. The data indicated that Ad-PRC1 promoted the growth of xenograft tumors by 20.3% (Fig 2A). 5-FU treatment significantly decreased the volume of tumors by 50.5%. However, the combination of 5-FU and Ad-PRC1 only reduced tumor growth by 33.1%, suggested that Ad-PRC1 infection increased the chemoresistance to 5-FU in vivo (P=0.008, Fig 2A). Of note, there was no obvious change in the body weights between the four groups (Fig 2B). The similar results were found in tumor weight detection (Fig 2C, Fig 2D).
PRC1 reversed the 5-FU-induced G2/M-phase arrest and regulated the expression of cell-cycle correlated molecules
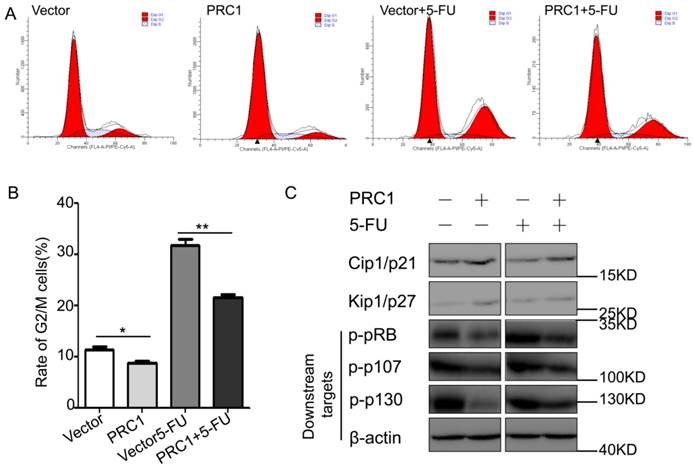
To investigate the potential mechanism by which PRC1 promoted tumor growth and increased the tumor resistance to 5-FU, we examined the cell cycle of cells in different groups and the expression of relevant molecules in cell cycle arrest. As shown in Fig 3A and 3B, 5-FU treatment induced an obvious G2/M-phase arrest compared with vector group (31.8±2.1% vs. 11.4±0.78, P=0.001). While combined with PRC1 overexpression, the percentage of G2/M-phase cell significantly decreased (31.8±2.1% vs. 21.6± 0.85%). These data suggested that PRC1 significantly abrogated the G2/M-phase arrest induced by 5-FU, which might contribute to the chemoresistance of PRC1. Furthermore, we detected expression level of the G2/M phase checkpoint proteins and the phosphorylation of downstream pRB molecules. The western blot data revealed that PRC1 reversed the effect of 5-FU which down-regulated the expression of Cip1/p21 and Kip1/P27 and triggered the phosphorylation of pRB, p107, p130(Fig 3C). These observations were consistent with data of cell cycle distribution.
PRC1 promoted the growth of HCC xenograft tumors and conferred resistance of tumors to chemotherapy in vivo. (A) The tumor volume was measured once every 5 days and calculated by the formula: length×width2×1/2. (B)The body weights of xenograft models with different treatment were examined once every 5 days. It was a surrogate marker for toxicity.(C)The representative pictures of nude mice xenograft models with different treatment. C1: Ad-Vector; C2:Ad-PRC1; C3: Ad-Vector+5-FU; C4: Ad-PRC1+5-FU. (D)Tumor weights in different groups.
PRC1 abrogated the G2/M-phase arrest and regulated Cip1/p21 and Kip1/p27 expression in HCC cells. (A) The cell cycle distribution in cells from different groups was analyzed by flow cytometry via PI staining. (B) The rate of G2/M phase cells from different groups was shown in histogram(*P<0.05, **P<0.01). (C) The expression levels of Cip1/p21, Kip1/p27 and the phos-pRB/p107/p130 protein levels were examined using western blot assay. β-actin was used as internal control.
Demographics and Clinical Features of the Patients With Hepatocellular Carcinoma Involved in Survival Analysis.
| Characteristics | PRC1 expression | P value | |
|---|---|---|---|
| Low expression(score<3) | High expression(score≥3) | ||
| Number of cases, n(%) | 99(44.6) | 123(55.4) | |
| Sex | |||
| Male | 70(31.5) | 85(38.3) | 0.911 |
| Female | 29(13.1) | 38(17.1) | |
| Age(year) | |||
| <50 | 48(21.6) | 50(22.5) | 0.302 |
| ≥50 | 51(23.0) | 73(32.9) | |
| TNM stage | |||
| I | 45(20.3) | 30(13.5) | |
| II | 24(10.8) | 31(14.0) | 0.004 |
| III | 25(11.3) | 48(21.6) | |
| IV | 5(2.2) | 14(6.3) | |
| Differentiation grade | |||
| Differentiated | 63(42.3) | 55(42.8) | |
| undifferentiated | 36(2.3) | 68(12.6) | 0.008 |
| Postoperative chemotherapy | |||
| Yes | 58(26.1) | 56(25.2) | 0.285 |
| No | 41(18.5) | 67(30.2) | |
| Serum carcinoembryonic antigen (ng/mL) | |||
| ≤2.62 | 45(20.3) | 30(13.5) | <0.001 |
| <2.62 | 54(24.3) | 93(41.9) | |
a5-Fluorouracil+oxaliplatin/cisplatin (without lymph node metastasis); 5-fluorouracil+oxaliplatin/cisplatin+paclitaxel (with lymph node metastasis).
Abbreviations: TNM, tumor-node-metastasis.
Significant association between up-regulation of PRC1 and clinical aggressiveness of HCC
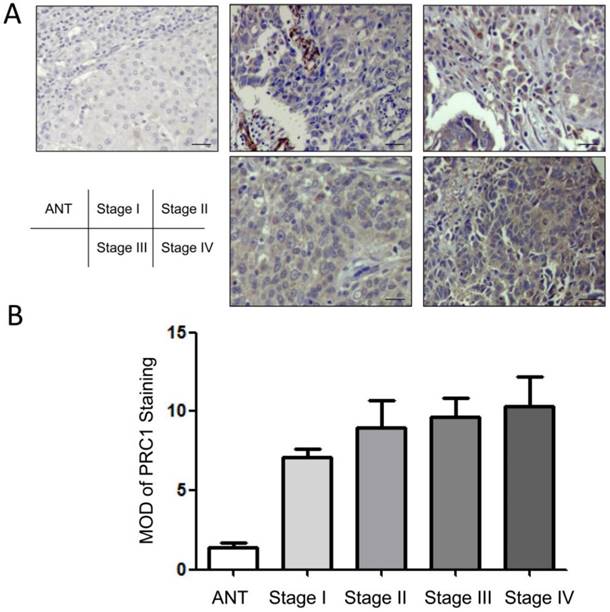
To further detect whether PRC1 up-regulation was associated with the clinical aggressiveness of HCC, 222 FFPE specimens were cut into 4μm sections and immunohistochemical stained. The results were summarized in Table 1. High level of PRC1 expression were present in cytoplasm of tumor cells of primary HCC. In contrast, PRC1 was slightly detectable in the adjacent non-cancerous tissues (Fig 4A). Quantitative analysis suggested that the MODs of PRC1 immunostaining in HCC specimens were higher than that in adjacent non-cancerous tissues (P<0.001; Fig 4B). Moreover, the expression of PRC1 was slightly lower in specimens from stage I and II than in those from stage III and IV HCC. But spearman correlation analysis indicated the expression of PRC1 was not associated with TNM stage (P=0.067). These results supported the hypothesis that PRC1 was involved in malignant transformation of HCC.
PRC1 expression in cancer predicted the unfavorable prognosis of HCC patients
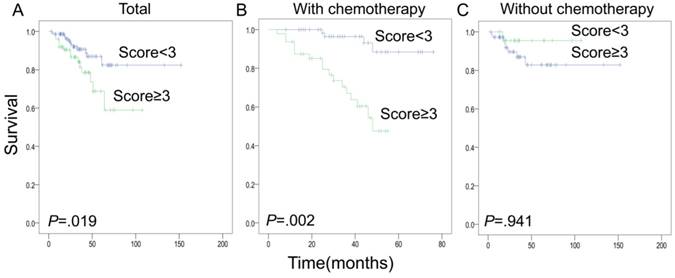
Demographic and clinicopathological variables of HCC patients included in survival analysis were presented in Table 1. High level of PRC1 expression in tumor cells was significantly correlated with an unfavorable postoperative survival (Fig 5). Furthermore, stratification analysis indicated that this correlation was only found in those patients who received 5-FU/platinum-based chemotherapy after curative surgery, but not in the patients without chemotherapy. The results of multivariate Cox regression analysis depicted the contribution of PRC1 expression and clinicopathological variables to HCC-specific survival (Table 2). High expression of PRC1 in tumor significantly predicted the unfavorable prognosis.
Expression pattern of PRC1 in adjacent non-cancerous tissues(ANT) and tumor tissues with different TNM stage. (A) Representative micrographs of PRC1 expression in histopathological sections which were examined by immunohistochemistry(IHC). Magnification: 200×; (B) The average MOD of PRC1 staining was drawn in histogram. Data was represented as mean±SD.
Kaplan-Meier analysis of postoperative survivals of HCC patients with different PRC1 expression levels in tumor specimens. Blue line: low PRC1 expression(immunostaining score:<3); Green line: high PRC1 expression(immunostaining score:≥3). (A)The curves for all the 222 patients; (B)Curves for patients with postoperative chemotherapy; (C) Curves for patients without chemotherapy.
Clinicopathological Variables and PRC1 expression in tumor specimens predicted postoperative survival of the 222 patients with hepatocellular carcinoma in the Cox proportional hazard models.
| Variables | Univariate Cox Analysis | Multivariate Cox Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 96%CI | P | HR | 95%CI | P | |
| Age,y(<50,≥50) | 2.264 | 1.689-3.521 | .003 | 2.137 | 1.596-3.287 | 0.005 |
| Sex(male vs. female) | 1.446 | 0.912-2.283 | 0.129 | |||
| TNM stage | 2.371 | 1.796-3.425 | <0.001 | 2.362 | 1.782-3.391 | <0.001 |
| Chemotherapy(yes vs. no) | 3.127 | 2.145-5.392 | 0.007 | |||
| PRC1 score(<3 vs, ≥3) | 4.942 | 1.591-15.347 | 0.006 | 4.458 | 1.418-14.019 | 0.011 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TNM, tumor-node-metastasis.
Discussion
To our knowledge, this study is the first one declare that PRC1 expression confer HCC cells the chemoresistance to 5-FU, inhibit 5-FU-induced apoptosis, abrogate 5-FU related G2/M phase arrest, promote the growth of xenograft tumors, and contribute to the unfavorable prognosis in HCC patients, especially for whom receive postoperative chemotherapy.
Currently, the development of drug resistance is the major factor for failure in chemotherapeutic intervention of HCC and relapse[15]. Therefore, conventional chemotherapeutic avenues achieve poor efficacy and show little benefit to survival in advanced HCC patients[16]. However, the potential mechanism is not so clear. MAPs are proteins calling too much attention from researchers for the functions in promoting microtubule stabilization and chemoresistance[8]. In the study, we found that PRC1, a novel MAP molecule, was screened from microarray database and proved that it was involved in HCC recurrence[9, 10]. Interestingly, the Huh-7 cells with PRC1 ectopic expression exhibited a significant resistance to 5-FU-induced growth inhibition and apoptosis (Fig 1). The dose-response effects reflect the actual role of PRC1 in chemoresistance.
Moreover, we detected the effect in chemoresistance of PRC1 in vivo. The data indicated that 5-FU treatment significantly decreased the volume of tumors by 50.5%. However, the combination of 5-FU and Ad-PRC1 only reduced tumor growth by 33.1%, suggested that intratumoral injected Ad-PRC1 infection increased the chemoresistance to 5-FU in vivo (P=0.008, Fig 2A). The similar results were found in tumor weight detection(Fig 2C, Fig 2D ). Taken together, our results demonstrated that PRC1 conferred the tumor cells chemoresistance to 5-FU both in vitro and in vivo. However, the underlying molecular mechanism should be explored.
Mounting evidences has shown that chemoresistance is correlated to mitotic checkpoint initiating, cell G2/M-phase arrest & apoptosis[17, 18]. Cip/p21 and Kip/p27 are negative regulators of cell cycle and inhibitors of cyclins, cyclin-dependent kinase(CDKs). They also play an pivotal role in the regulation of cytotoxic-agent resistance[19]. While receive anti-proliferative signals, the downstream molecules(pRB, p107, p130) of Cip/p21 and Kip/p27 undergo phosphorylations during the progression through S and G2/M, and the de-phosphorylation of pRBs is essential for cells to pass the mitosis[20] . Therefore, to elucidate the mechanisms by which PRC1 could influence the chemoresistance of HCC cells, we extended our investigations to its effects on the cell-cycle distribution and the expression of these cell-cycle-regulatory molecules. Since pRb is phosphorylated and hyperphosphorylated in S and G2/M phase, respectively, we also examined the phosphorylation status of pRBs in HCC cells from different groups. Surprisingly, we found that the ectopic expression of PRC1 abrogated the G2/M phase arrest in 5-FU treated Huh-7 cells(31.8±2.1% to 21.6± 0.85%)(Fig 3A and Fig 3B). In addition, we also demonstrated that up-regulated expression of cell cycle regulators was triggered by PRC1(Fig 3C). The phosphorylation status of downstream pRBs were subsequently altered. These evidences were consistent with changes of the cell cycle distribution. Our findings confirmed the viewpoint that PRC1 enhanced the chemoresistance of HCC cells to 5-FU by abrogating the agent-induced G2/M-phase arrest. The effect of PRC1 on cell cycle distribution was mainly due to the up-regulation of CDK inhibitor-Cip1/p21 and Kip1/P27, and subsequently inhibition of the phosphorylation of pRBs.
Since PRC1 is an essential MAP molecule which confer chemoresistance of HCC cells, the association between PRC1 expression and prognosis should be evaluated. We included 222 HCC patient with intact clinicopathological informations, follow-up and PRC1 immunohistochemical data in the survival analysis. The results suggested that PRC1 expression in HCC tissues was significantly associated with unfavorable postoperative survival, especially for the patients who received postoperative chemotherapy (Fig 5). This effect was independent of TNM stage (Table 2). Because PRC1 expression increases chemoresistance, chemotherapy may screen a subset of HCC cells with high PRC1 expression. These cells might be major populations of cancer relapse. Targeting PRC1 pathway might improve the effect of postoperative chemotherapy. Thus, this study provides a new candidate therapeutic target to improve HCC prognosis.
In conclusion, our findings demonstrated that higher expression of PRC1 in HCC cells contributed to the chemoresistance to 5-FU both in vitro and in vivo, reversal of 5-FU-induced G2/M-phase arrest, and the unfavorable prognosis in HCC patients, especially for whom received postoperative chemotherapy. This study provided a novel predictive marker for postoperative prognosis of HCC. Targeting PRC1 might be an effective option in HCC combined chemotherapy intervention to improve the survival of patients.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No.81402090 to W.Y.).
Author contribution
Y.W., F.S., S.X. and J.H. designed the experiments and analyzed data. Y.W., F.S., and G.X. performed all the experiments. P.X., N.Z., Y.Y. and S.S. wrote the manuscript and prepared the figures. All authors reviewed and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108
2. Marquardt JU, Thorgeirsson SS. SnapShot: Hepatocellular carcinoma. Cancer Cell. 2014;25:550 e1
3. Ma W, Sze KM, Chan LK, Lee JM, Wei LL, Wong CM. et al. RhoE/ROCK2 regulates chemoresistance through NF-kappaB/IL-6/ STAT3 signaling in hepatocellular carcinoma. Oncotarget. 2016
4. Jiang W, Jimenez G, Wells NJ, Hope TJ, Wahl GM, Hunter T. et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol Cell. 1998;2:877-85
5. Subramanian R, Wilson-Kubalek EM, Arthur CP, Bick MJ, Campbell EA, Darst SA. et al. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell. 2010;142:433-43
6. Wei W, Birrer MJ. Spleen Tyrosine Kinase Confers Paclitaxel Resistance in Ovarian Cancer. Cancer Cell. 2015;28:7-9
7. De Angelis PM, Svendsrud DH, Kravik KL, Stokke T. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20
8. Poruchynsky MS, Giannakakou P, Ward Y, Bulinski JC, Telford WG, Robey RW. et al. Accompanying protein alterations in malignant cells with a microtubule-polymerizing drug-resistance phenotype and a primary resistance mechanism. Biochem Pharmacol. 2001;62:1469-80
9. Wang SM, Ooi LL, Hui KM. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin Cancer Res. 2011;17:6040-51
10. Chen J, Rajasekaran M, Xia H, Zhang X, Kong SN, Sekar K. et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/beta-catenin signalling pathway. Gut. 2016
11. Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J. et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236-47
12. Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J. et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497-506
13. Xu WP, Yi M, Li QQ, Zhou WP, Cong WM, Yang Y. et al. Perturbation of MicroRNA-370/Lin-28 homolog A/nuclear factor kappa B regulatory circuit contributes to the development of hepatocellular carcinoma. Hepatology. 2013;58:1977-91
14. Yin C, Wang PQ, Xu WP, Yang Y, Zhang Q, Ning BF. et al. Hepatocyte nuclear factor-4alpha reverses malignancy of hepatocellular carcinoma through regulating miR-134 in the DLK1-DIO3 region. Hepatology. 2013;58:1964-76
15. Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H. et al. A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget. 2015;6:31927-43
16. Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA. et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101:578-86
17. Zhang Y, Sriraman SK, Kenny HA, Luther E, Torchilin V, Lengyel E. Reversal of chemoresistance in ovarian cancer by co-delivery of a P-glycoprotein inhibitor and paclitaxel in a liposomal platform. Mol Cancer Ther. 2016
18. Jia J, Wang Z, Cai J, Zhang Y. PMS2 expression in epithelial ovarian cancer is posttranslationally regulated by Akt and essential for platinum-induced apoptosis. Tumour Biol. 2016;37:3059-69
19. Gregorc V, Ludovini V, Pistola L, Darwish S, Floriani I, Bellezza G. et al. Relevance of p53, bcl-2 and Rb expression on resistance to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2003;39:41-8
20. King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563-71
Author contact
![]() Corresponding authors: Shi-Ying Xuan, Department of Gastroenterology, Qingdao Municipal Hospital, Medical College, Qingdao University, Qingdao, Shandong, China. E-mail: dxyxyncom; Yu Wang, Department of Gastroenterology, Rizhao People's Hospital, Rizhao, Shandong, China. E-mail: wlwangyu1104com; Ying Wang, Department of Cardiovascular Surgery of the First Affiliated Hospital& Institute for Cardiovascular Science, Soochow University, Suzhou, Jiangsu, China. Email: wangying8108edu.cn; and Jing He, Department of Pediatric Surgery, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, China. E-mail: hejing27sysu.edu.cn.
Corresponding authors: Shi-Ying Xuan, Department of Gastroenterology, Qingdao Municipal Hospital, Medical College, Qingdao University, Qingdao, Shandong, China. E-mail: dxyxyncom; Yu Wang, Department of Gastroenterology, Rizhao People's Hospital, Rizhao, Shandong, China. E-mail: wlwangyu1104com; Ying Wang, Department of Cardiovascular Surgery of the First Affiliated Hospital& Institute for Cardiovascular Science, Soochow University, Suzhou, Jiangsu, China. Email: wangying8108edu.cn; and Jing He, Department of Pediatric Surgery, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, China. E-mail: hejing27sysu.edu.cn.

 Global reach, higher impact
Global reach, higher impact