3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2017; 8(4):507-512. doi:10.7150/jca.17644 This issue Cite
Research Paper
Efficacy of Cabazitaxel Treatment in Metastatic Castration Resistant Prostate Cancer in Second and Later Lines. An Experience from Two German Centers
1. Department of Medical Oncology, National Center for Tumor Diseases (NCT), Heidelberg University Hospital, Im Neuenheimer Feld 460, 69120 Heidelberg, Germany;
2. Department of Urology, Heidelberg University Hospital, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany;
3. Onkologische Schwerpunktpraxis Heidelberg, Kurfürstenanlage 34, 69115 Heidelberg, Germany;
4. Department of Urology, Klinikum Nürnberg, Paracelsus Medical University, Prof.-Ernst-Nathan-Str. 1, 90419 Nürnberg, Germany;
5. Section of Molecular Urooncology, Department of Urology, Heidelberg University Hospital, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany.
Received 2016-9-20; Accepted 2016-12-23; Published 2017-2-11
Abstract
Purpose: Several new treatment options for patients with metastatic castration resistant prostate cancer (mCRPC) have been approved within the last years - among them cabazitaxel (CAB), abiraterone acetate, enzalutamide, and radium-223. The aim of this study was to assess factors predictive for efficacy of CAB.
Methods: We analyzed all patients with mCRPC treated with CAB at our institutions between 2011 and 2016. Data were retrieved retrospectively from the electronical patient chart.
Results: 69 patients received CAB (26.1% 2nd line, 36.2% 3rd line, 37.3% >3rd line). Median overall survival (OS) on CAB was 10.0 months (95%CI 7.1-12.9). Median progression free survival (PFS) on CAB was 3.9 months (95%CI 3.0-4.8). There were no differences in OS and PFS regarding treatment line of CAB (2nd vs. higher; 2nd/3rd vs. higher). Duration of remission on 1st line treatment (> 6 months vs. </= 6 months) was associated with a longer PFS with subsequent CAB treatment (4.1 months vs. 3.0 months (95%CI 3.0-5.2; 2.2-3.8); p=0.021). Patients with visceral metastases had a shorter PFS (3.0 months; 95%CI 2.6-3.3) and OS (8.7 months; 95%CI 5.9-11.5) on CAB compared to patients who had bone and/or lymph node lesions only (PFS: 5.8 months; 95%CI 3.2-8.4; p=0.014; OS: 11.7 months; 95%CI 7.5-15.9; p=0.042).
Conclusions: Results from our patient cohort suggest that a longer PFS to any 1st line treatment for mCRPC is correlated with a longer PFS to CAB for any later line treatment. Patients with nodal and bone metastases only had a significantly superior PFS and OS with CAB treatment than patients with visceral metastases.
Keywords: Cabazitaxel, prostate cancer, sequencing therapy, survival, castration resistance.
Purpose
Patients that suffer from metastatic castration resistant prostate cancer (mCRPC) had few treatment options for a long time [1]. During the last decade major progress has been made extending the treatment options considerably. New generation anti-hormonal agents (NAA; abiraterone (ABI), enzalutamide (ENZA)) new radiotherapeutics (alpharadin; radium-223) and a new taxan chemotherapy (cabazitaxel (CAB)) have all been approved and shown to be active after failure of docetaxel (DOC) as a 2nd line therapy [2,3,4,5,6]. However, limited data are available to differentiate patients that may benefit rather from chemotherapy with CAB than from a NAA post DOC. Moreover, none of these substances have been investigated in 3rd or 4th line within a prospective randomized clinical trial, and there is concern regarding acquired cross resistances among them. Nevertheless, in real-world oncology many patients receive more than two treatment lines aiming at a cumulative survival benefit that has yet to be confirmed. Prospective trials with approved substances are rarely ever conducted. Hence, retrospective analyses might help to improve decision making and treatment processes for this relevant patient group.
The objective of this study was to analyze all patients from our comprehensive cancer center and private practice who received CAB either in 2nd line or in later lines for clinical parameters associated with a response to CAB.
Methods
In this retrospective observational study all 69 patients with mCRPC treated with CAB at the National Center of Tumor Diseases (NCT) and a private practice were identified and their records accessed through the electronic patients charts [7]. The project was approved by the local ethics committee.
Clinical parameters assessed included Gleason score (GS), tumor stage, location of metastases, time to castration resistance on androgen ablation therapy, type of 1st line therapy, type of 2nd line therapy, response to 1st line therapy and duration of response (prostate specific antigen (PSA) and radiographic) as well as response and duration of response to later lines of CAB.
Progression free survival (PFS) was evaluated using the Prostate Cancer Working Group 2 (PCWG2) criteria [8]. Side effects were classified according to CTCAE dictionary version 4.0. Overall survival (OS) was calculated from the date of start of systemic anti-hormonal treatment and the start of CAB treatment to the date of death or date of last follow-up (last assessed on March 2016). Survival and progression were calculated using Kaplan-Meier estimates and compared using log-rank tests. Associations between factors were evaluated using non-parametric Kendall-tau or Pearson correlation. Primary outcomes were the OS as well as the PFS since initiation of CAB. Secondary outcomes were PFS on 1st line treatment as well as correlation analyses between treatment factors. Statistical analyses were conducted using the SPSS v21 software.
Results
The final study population consisted of 69 patients that started systemic anti-hormonal LHRH analogue treatment between February 1998 and January 2015. Most of the patients received DOC as 1st line therapy (n=52, 75.4%). Other 1st line therapies included ABI (n=9), ENZA (n=4), ipilimumab (n=1, as part of a clinical trial), and PSMA ligands (n=3, as part of a clinical trial). In 2nd line, patients received ABI (n=31), CAB (n=18), DOC (n=12), ENZA (n=5), RNA vaccine (n=1, as part of a clinical trial), and mitoxantrone (n=2). CAB was applied as 3rd line treatment in 25 patients, as 4th line therapy in 19 patients, as 5th line in 5 patients, and as 6th line in 2 patients.
CAB was administered according to the protocol that was established by the Phase-III TROPIC trial in the majority of patients [1]. However, 17.3 % of patients received an upfront dose-reduction (i.e. 20% = 20 mg/m² qd22, n=9; ~30% = 17.5-18 mg/m² qd22, n=3) due to reduced general condition (ECOG 2 or ECOG 1 plus bone marrow dysfunction). Three patients were treated biweekly analogue the Prosty II trial protocol [9]. Six patients received CAB on a weekly scheme. In four cases dose reductions were necessary after cycles 1 (n=2), 2 (n=1) and 6 (n=1) due to bicytopenia, respectively. All patients received CAB in 4th line. In 3 patients, CAB regime was changed from three-weekly to weekly after cycle 1 due to bicytopenia. Those patients had received CAB in 3rd, 4th and 6th treatment line. Treatment was generally well tolerated with an average of 5 cycles (range 1-11) and a treatment duration of 3.6 months (range 0.4-17.0). Treatment was discontinued in most cases either due to progressive disease or when anticipated cycles of CAB had been applied. In one case CAB treatment was terminated due to patient preference. In four patients treatment was discontinued due to toxicity (neutropenic fever CTC 4° (n=2); acute renal insufficiency CTC 4° (n=1); peripheral sensory and motor neuropathy CTC 3° in an 88-years old individual (n=1)). Three CTC 5° side effects were noted: Two patients died in neutropenic sepsis (84-years old patient on cycle 1 day 11; 75-years old patient on cycle 2 day 11). A 71-years old patient died due to spontaneous subdural hematoma as a result of CAB-induced thrombocytopenia. Three patients are still on treatment. Patient characteristics are summarized in Table 1.
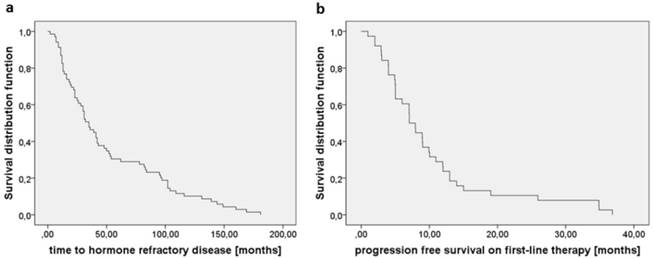
Median time between the diagnosis of prostate cancer and the initiation of androgen deprivation was 2.0 months (95%CI 1.0-7.9). Median time to castration resistance was 35.0 months for all investigated patients (95%CI 26.0-43.0, Fig. 1a). Median PFS on 1st line treatment was 7.0 months (95% CI 5.5-8.5, Fig. 1b). There were no significant differences regarding PFS on 1st line treatment between DOC vs. any other treatment type or GS 6-7 vs. 8-10.
Patient Characteristics.
| Parameter | No. | % (Range) |
|---|---|---|
| Total number of patients | 69 | 100 |
| Age at diagnosis [years], median | 62 | (46 - 81) |
| Age at CAB [years], median | 69 | (51 - 88) |
| Gleason Score at first diagnosis | ||
| 6-7 | 27 | 39.1 |
| 8-10 | 42 | 60.9 |
| Clinical nodal status at first diagnosis | ||
| N0 | 32 | 46,4 |
| N1 | 33 | 47,8 |
| missing | 4 | 5,8 |
| Metastatic spread (prior to CAB treatment) | ||
| bone metastases | 59 | 85.5 |
| peritoneal metastases | 4 | 5.8 |
| lung metastases | 14 | 20.3 |
| liver metastases | 24 | 34.8 |
| brain metastases | 3 | 4.3 |
| adrenal gland metastases | 8 | 11.6 |
| CAB treatment cycles, median | 5 | (1-11) |
| Duration on CAB, median | 3.6 | (0.4-17.0) |
| CAB regime | ||
| 25 mg/m², qd21 | 42 | 60.8 |
| 20 mg/m², qd21 | 9 | 13.0 |
| 17.5 -18.0 mg/m², qd21 | 3 | 4.3 |
| 16 mg/m², qd15 | 3 | 4.3 |
| 10 mg/m², qd8 | 6 | 8.7 |
| unknown | 6 | 8.7 |
| Type of first line treatment | ||
| Docetaxel | 52 | 75.4 |
| Abiraterone | 9 | 13.0 |
| Enzalutamid* | 4 | 5.8 |
| PSMA ligand* | 3 | 4.3 |
| Ipilimumab (within clinical trial) | 1 | 1.4 |
| Type of 2nd line treatment | ||
| Docetaxel | 12 | 17.4 |
| Abiraterone | 31 | 44.9 |
| Enzalutamid | 5 | 7.2 |
| CAB | 18 | 26.1 |
| Mitoxantron | 2 | 2.9 |
| RNA vaccine* (within clinical trial) | 1 | 1.1 |
| CAB sequence | ||
| 2nd line | 18 | 26.1 |
| 3rd line | 25 | 36.2 |
| 4th line | 19 | 27.5 |
| 5th line | 5 | 7.2 |
| 6th line | 2 | 2.9 |
| Mean PSA at initial diagnosis | 96.2 ng/ ml | (0.1-900.0) |
| Mean PSA at time point of castration resistance | 68.5 ng/ ml | (0.6-355.0) |
| Mean PSA at time point of CAB begin | 453.0 ng/ml | (0.0-5102.0) |
| Mean PSA at time point of progressive disease post-CAB | 478.8 ng/ml | (0.0-5074.0) |
| PSA response in treatment period | ||
| ≥ 30% | 24 | 34.8 |
| ≥ 50% | 20 | 29.0 |
| ≥ 90% | 4 | 5.8 |
Abbreviations: CAB: cabazitaxel; PSA: prostate specific antigen; PSMA: prostate specific membrane antigen.
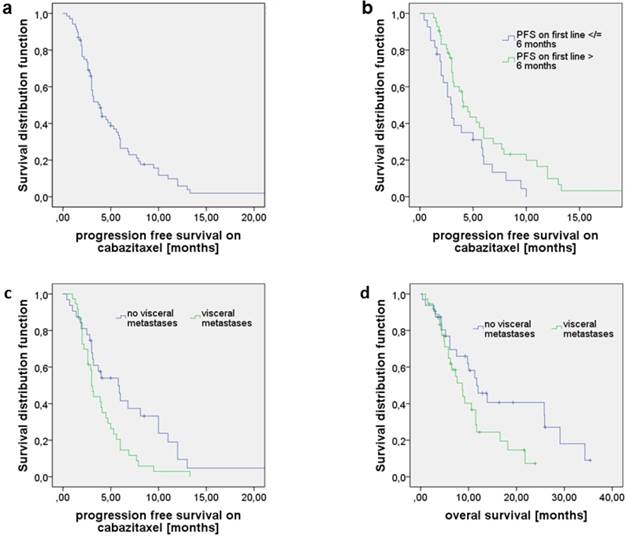
Median OS since start of CAB treatment was 10.0 months (95% CI 7.0-12.9). Median PFS on CAB treatment was 3.9 months (95% CI 3.0-4.8, Fig. 2a). There were no differences on OS and PFS on CAB stratified for GS (6-7 vs. 8-10), age at diagnosis, age at begin of CAB treatment, type of 1st line therapy (DOC vs. any other), line of CAB (2nd vs. any other; 2nd/ 3rd vs. any other), and absolute PSA prior to CAB treatment (analyzed for percentile ranks 50; 66.7; 75) (data not shown). A PSA decline ≥ 50% was seen in n=20 patients receiving CAB (29.0%). A PSA reduction of at least 30% was noticed in n=24 patients (24.8%). PSA decline ≥ 50% on CAB showed a prolonged OS (16.6 months vs. 9.2 months; 95% CI 6.0-27.3; 6.2-12.2; p=0.087) and PFS (6.9 vs. 3.0 months, 95% CI 3.7- 10.2; 2.7-3.3; p= 0.09), however not statistically significant. Statistical significance was reached for the larger cohort with a PSA decline of at least 30% for OS (16.6 vs. 8.8 months; 95%CI 6.5-26.7; 6.2-11.4; p=0.039) and PFS (6.9 vs. 3.0 months; 95%CI 4.5-9.3; 2.7-3.3; p=0.03).
When stratified for PFS on 1st line therapy (≤ 6 months vs. > 6 months), patients with a PFS > 6 months had a significantly longer PFS on CAB in any later treatment line that was 4.1 months vs. 3.0 months (95%CI 3.0-5.2; 2.2-3.8; p=0.021, Fig. 2b). No associations were noted between OS and duration of remission on 1st treatment line. However, there was a trend towards longer OS on CAB treatment for patients that remained on remission on DOC > 6 months (10.6 vs. 7.6 months; 95% CI 7.8-13.4; 4.4-10.8; p=0.051).
Patients with visceral metastases at time of CAB initiation had a significantly shorter PFS on CAB reaching 3.0 months vs. 5.8 months (95%CI 2.7-3.3; 3.2-8.4; p=0.014, Fig. 2c) and a shorter OS compared to those who only had bone or/and lymph node lesions (8.7 months vs. 11.7 months, 95% CI 5.9-11.5; 7.5-15.9; p=0.042, Fig. 2d) whereas PFS on 1st line treatment did not differ significantly between those two groups.
Discussion
Therapeutic options for mCRPC patients have been greatly expanded within the last couple of years. Beside NAAs, with CAB a novel taxane has also entered clinical practice [10,11]. CAB has been approved for the treatment of mCRPC in the post-docetaxel setting as a 2nd line treatment and is currently investigated within the FIRSTANA trial as 1st line treatment option at different dose levels. However, in the real world CAB is often also used post ABI or ENZA as a 3rd or 4th line therapy being efficient in those settings [12,13,14,15]. Retrospective single and multicenter experiences as well as meta-analyses have come to contradicting results for the optimal sequence of medication. While some showed no significant differences between the different agents used in 3rd or 4th line [16], others pointed to a survival benefit when applying the sequence DOC-CAB-ABI [14,17,18] or the sequence of CAB-NAA or vice versa compared to NAA-NAA post DOC [19]. However, no prospective randomized clinical trial has investigated treatment outcomes beyond 2nd line and for different sequences. In addition, in the aftermath of the CHAARTED and STAMPEDE trials more and more patients will have received DOC in the hormone-sensitive setting raising new questions of optimal therapeutic approaches upon the development of castration resistant disease. In our retrospective cohort patients with a PFS longer than 6 months on 1st line therapy had a significantly longer PFS on CAB irrespective of the line of treatment. Interestingly, it has been shown before, that cross resistance may exist between new anti-androgens and taxanes which is in accordance with our data [20,21].
a) Time between initiation of systemic LHRH treatment and the development of castration resistant disease was 35.0 months. b) PFS on 1st line treatment independent of treatment type was 7.0 months.
a) Progression free survival (PFS) on cabazitaxel (CAB) as 2nd or later line treatment; median PFS was 3.9 months. b) PFS on CAB stratified for PFS on 1st line (≤ 6 months vs. > 6 months). Patients with PFS1st line > 6 months had a significantly longer PFS on CAB in any later treatment line (4.1 months vs. 3.0; p=0.021). c) PFS of patients with visceral metastases was 3.0 compared to 5.8 months when no visceral metastases were present (p=0.014). d) Overall survival (OS) of patients with visceral metastases was 8.7 compared to 11.7 months in the absence of visceral metastases (p=0.042).
A high GS (as defined as 8-10) was not predictive for response to CAB as previously reported [22]. While previously published studies showed a correlation between longer duration of remission on first line DOC for higher GS [21,23] we could not detect any such correlation for CAB in our cohort. We further found no correlation between the absolute level of PSA pre-CAB. While PSA increases during treatment for CRPC may be flare up phenomena and are not generally associated with radiographic progression [4], a PSA decline is in most studies associated with a better survival. We could also demonstrate that statistical significance for OS benefit was already reached with a PSA decline of at least 30%.
Large pooled retrospective analyses have revealed that the existence of visceral metastases is a negative prognostic factor associated with reduced OS of 13.3 months for patients with liver metastases and 19.4 months for patients with lung metastases [24,25]. In our cohort, OS on CAB for patients with all types of visceral metastases was 8.7 months only. Interestingly, we also noted a significant difference in PFS on CAB in favor for patients with bone and/or bone and lymph node metastases compared to those with visceral metastases in our cohort (5.8 vs. 3.0 months). A difference in PFS was not observed in 1st line treatment when stratified for visceral metastases. One explanation for the poor response of patients with visceral metastases could be that clonal selection associated with visceral spreading is associated with a more therapy-resistant phenotype, e. g neuroendocrine subtypes [26,27]. Biopsies from visceral metastatic sites might help to further genetically characterize these clones and search for other treatment protocols. More prospective data are required to determine if CAB is a meaningful choice for mCRPC with visceral involvement.
We reported three grade 5 toxicities and several treatment discontinuations and dose modifications showing that CAB has substantial toxicity in this elderly and frail population. It should be noted that lower doses are equally effective with lower toxicity [28, 29] and should be considered as initial dose level.
Limitations to our study are its retrospective character, dual center data and small sample size that may have introduced a bias. These factors might explain the inability to detect survival differences due to to inadequate power in some analyses.
Clinical Practice Points
The optimal treatment sequence for agents in mCRPC is still unknown. Prospective clinical trials are needed.
Conclusions
Sequencing of new substances for mCRPC remains a subject of debate. CAB is approved for second line therapy following DOC. However, it is also used following new anti-hormonal treatments or in later lines. In our analysis CAB seems to be active in all these indications, especially if the tumor was sensitive to any 1st line treatment, irrespective of the line of treatment when CAB was being deployed. However, patients with visceral metastases had a significantly shorter OS and PFS on CAB. Prospective studies are needed to identify the optimal treatment sequence for patients with mCRPC.
Abbreviations
ABI: abiraterone; CAB: cabazitaxel; DOC: docetaxel; ENZA: enzalutamide; GS: Gleason score; mCRPC: metastatic castration resistant prostate cancer; NAA: new generation anti-hormonal agent; OS: overall survival; PFS: progression free survival; PSA: prostate specific antigen; PSMA: prostate specific membrane antigen
Acknowledgements
The NCT is supported by the German Cancer Research Center (DKFZ), the University Hospital Heidelberg in cooperation with the Medical Faculty Heidelberg and by the German Cancer Aid (Deutsche Krebshilfe). We thank the NCT registry for providing information on survival dates.
Authors' contributions
D Debatin: Data collection
S Duensing: Data collection
S Fuxius: Data collection
C Grüllich: Protocol/project development, Data collection, Data analysis, Manuscript writing/editing
B Hadaschik: Data collection
M Hohenfellner: Data collection
D Jäger: Data collection
A Karcher: Data collection
S Pahernik: Data collection
C Spath: Data collection
S Vallet: Data collection, Data analysis
S Zschäbitz: Protocol/project development, Data collection, Data analysis, Manuscript writing
Ethics Committee Approval and Patient Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients provided written informed consent. Any information connected with the identity of individual subjects was excluded from this study.
Consent for publication was approved by the local ethics committee. All authors have read and agreed on the current version of the manuscript.
Availability of data and material
Original data and material are completely available upon requested from the corresponding author.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Tannock IF, de Wit R, Berry WR. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512
2. de Bono JS, Oudard S, Ozguroglu M. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-1154
3. de Bono JS, Logothetis CJ, Molina A. et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005
4. Fizazi K, Scher HI, Molina A. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983-992
5. Scher HI, Fizazi K, Saad F. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197
6. Parker C, Nilsson S, Heinrich D. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-223
7. Huber J, Herpel E, Jakobi H. et al. Two decades' experience with a prospective biobank for urologic oncology: research, clinical care, and the patients' view. Urol Oncol. 2013;31:990-996
8. Scher HI, Halabi S, Tannock I. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148-1159
9. Lehtinen P-LIK. Biweekly cabazitaxel as post-docetaxel treatment for metastatic castration resistant prostate cancer (mCRPC): Findings from an early safety analysis of the Prosty II trial. J Clin Oncol. 2015:33
10. Omlin A, Pezaro CJ, Zaidi S. et al. Antitumour activity of abiraterone and diethylstilboestrol when administered sequentially to men with castration-resistant prostate cancer. Br J Cancer. 2013;109:1079-1084
11. Bianchini D, Lorente D, Rodriguez-Vida A. et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78-84
12. Sella A, Sella T, Peer A. et al. Activity of cabazitaxel after docetaxel and abiraterone acetate therapy in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2014;12:428-432
13. Pezaro CJ, Omlin AG, Altavilla A. et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol. 2014;66:459-465
14. Wissing MD, Coenen JL, van den Berg P. et al. CAST: A retrospective analysis of cabazitaxel and abiraterone acetate sequential treatment in patients with metastatic castrate-resistant prostate cancer previously treated with docetaxel. Int J Cancer. 2015;136:E760-772
15. Al Nakouzi N, Le Moulec S, Albiges L. et al. Cabazitaxel Remains Active in Patients Progressing After Docetaxel Followed by Novel Androgen Receptor Pathway Targeted Therapies. Eur Urol. 2015;68:228-235
16. Caffo O, De Giorgi U, Fratino L. et al. Clinical Outcomes of Castration-resistant Prostate Cancer Treatments Administered as Third or Fourth Line Following Failure of Docetaxel and Other Second-line Treatment: Results of an Italian Multicentre Study. Eur Urol. 2015;68:147-153
17. Sonpavde G, Bhor M, Hennessy D. et al. Sequencing of Cabazitaxel and Abiraterone Acetate After Docetaxel in Metastatic Castration-Resistant Prostate Cancer: Treatment Patterns and Clinical Outcomes in Multicenter Community-Based US Oncology Practices. Clin Genitourin Cancer. 2015;13:309-318
18. Angelergues A. Prognostic factors of survival in patients with metastatic castration resistant prostate cancer (mCRPC) treated with cabazitaxel: sequencing might matter. J Clin Oncol. 2013:31 (Suppl. 6)
19. Maines F, Caffo O, Veccia A. et al. Sequencing new agents after docetaxel in patients with metastatic castration-resistant prostate cancer. Crit Rev Oncol Hematol. 2015;96:498-506
20. van Soest RJ, de Morree ES, Kweldam CF. et al. Targeting the Androgen Receptor Confers In Vivo Cross-resistance Between Enzalutamide and Docetaxel, But Not Cabazitaxel, in Castration-resistant Prostate Cancer. Eur Urol. 2015;67:981-985
21. Hofner T, Vallet S, Hadaschik BA. et al. Docetaxel followed by abiraterone in metastatic castration-resistant prostate cancer: efficacy and predictive parameters in a large single center cohort. World J Urol. 2015;33:833-839
22. Buonerba C, Pond GR, Sonpavde G. et al. Potential value of Gleason score in predicting the benefit of cabazitaxel in metastatic castration-resistant prostate cancer. Future Oncol. 2013;9:889-897
23. van Soest RJ, de Morree ES, Shen L. et al. Initial biopsy Gleason score as a predictive marker for survival benefit in patients with castration-resistant prostate cancer treated with docetaxel: data from the TAX327 study. Eur Urol. 2014;66:330-336
24. Halabi S, Kelly WK, Ma H. et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J Clin Oncol. 2016;34:1652-9
25. Halabi S, Lin CY, Small EJ. et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105:1729-1737
26. Beltran H, Prandi D, Mosquera JM. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298-305
27. Gururajan M, Cavassani KA, Sievert M. et al. SRC family kinase FYN promotes the neuroendocrine phenotype and visceral metastasis in advanced prostate cancer. Oncotarget. 2015;6:44072-44083
28. Sartor AO, Oudard S, Sengelov L. et al. Cabazitaxel vs docetaxel in chemotherapy-naive (CN) patients with metastatic castration-resistant prostate cancer (mCRPC): A three-arm phase III study (FIRSTANA). J Clin Oncol. 2016;34(Suppl):Abstr5006
29. De Bono JS, Hardy-Bessard AC, Kim CS. et al. Phase III non-inferiority study of cabazitaxel (C) 20 mg/m2 (C20) versus 25 mg/m2 (C25) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel (D). J Clin Oncol. 2016;34(Suppl):Abstr5008
Author contact
![]() Corresponding author: Carsten Grüllich, MD, PhD, Department of Medical Oncology, National Center for Tumor Diseases (NCT), Heidelberg University Hospital, Im Neuenheimer Feld 460,69120 Heidelberg, Germany. Phone: +49 6221 5637125; Fax: +49 6221 565318; E-Mail: carsten.gruellichuni-heidelberg.de.
Corresponding author: Carsten Grüllich, MD, PhD, Department of Medical Oncology, National Center for Tumor Diseases (NCT), Heidelberg University Hospital, Im Neuenheimer Feld 460,69120 Heidelberg, Germany. Phone: +49 6221 5637125; Fax: +49 6221 565318; E-Mail: carsten.gruellichuni-heidelberg.de.

 Global reach, higher impact
Global reach, higher impact