3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(14):2110-2116. doi:10.7150/jca.16211 This issue Cite
Research Paper
Incidence and predictors of Bone Metastases (BM) and Skeletal-Related Events (SREs) in Small Cell Lung Cancer (SCLC): A Swiss patient cohort
1. Department of Internal Medicine, Medical Oncology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland
2. Lung Tumor Center, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland
Received 2016-5-18; Accepted 2016-8-14; Published 2016-10-23
Abstract
Objectives: Bone metastases (BM) and skeletal-related events (SREs) are frequent complications in patients with lung cancer. Whereas in non-small-cell lung cancer (NSCLC) incidence, prognostic impact, and risk factors are well established, there is only little knowledge in patients with small cell lung cancer (SCLC). We retrospectively evaluated the incidence of BM, SRE and their treatment in a SCLC patient cohort treated at our hospital. We further assessed the role of Lactate Dehydrogenase (LDH), a possible predictor of BM development in SCLC patients.
Materials and Methods: We retrospectively analyzed patients with the diagnosis of SCLC for BM, SRE, overall treatment patterns, outcome and established prognostic parameters by record review. The prognostic role of LDH was tested using univariate longitudinal regression analysis.
Results: We identified 92 consecutive patients with SCLC diagnosed between 2000 and 2010 at our institution. Overall, 36.9% presented with BM at first diagnosis. Median time to BM from first diagnosis was 14.8 months (range) in limited disease (LD) and 0.9 months (range) in extensive disease (ED). The overall incidence of SRE was 18.4%. Only 19.6% of patients with BM were initially treated with bisphosphonates.
Conclusions: Elevated LDH, as well as age ≥75 years were independent predictors for BM development in SCLC patients. Although SREs are relevant complications in SCLC, early antiresorptive treatment of BM to reduce the risk of SREs was rare. LDH served as a predictive factor for BM development in our SCLC cohort and therefore should be taken into account in future randomized controlled trials.
Keywords: Bone metastases, Skeletal-related event, Small Cell Lung Cancer, LDH, Predictive factor
1. Introduction
Small-cell lung cancer (SCLC) accounts for about 15-20% of all lung cancers and has a dismal prognosis [1]. According to the Veteran`s Administration Lung Cancer Study Group in the United States [2] SCLC is traditionally classified into two stages, depending on the extent of disease: Limited disease (LD) and extensive disease (ED). In LD, diagnosed in about 30% of patients with SCLC, cancer affects one hemithorax plus regional lymph nodes. 70% of SCLC patients are however diagnosed with ED, where the tumor extends beyond boundaries of a single radiation field. Distant metastases involve mainly brain, liver and bone [3].
Bone metastases (BM) are painful and often associated with complications, so-called skeletal-related events (SREs), defined as pathologic fracture, spinal cord compression, the need of radiotherapy or surgery to the bone and hypercalcemia of malignancy (HCM) [4-6]. The development of SREs inversely correlates with quality of life in affected patients. Therefore, prevention strategies and early treatment are currently state of the art in the palliative treatment concept of BM [7,8].
Antiresorptive drugs (e.g. the intravenous bisphosphonates zoledronic acid (ZA), bondronate and pamidronate) reduce SREs in solid cancer patients with BM, including lung cancer [6,9-11]. Moreover, it has been shown that bisphosphonates can provide pain relief for BM independent of SREs and cancer type [12]. More recently, the fully human anti-RANKL monoclonal antibody denosumab was shown to be non-inferior to ZA in the prevention of SREs in solid cancers as well as multiple myeloma [4,13,14] and therefore became an alternative to ZA use. Furthermore, in an exploratory analysis, denosumab was associated with improved overall survival compared with ZA, in patients with metastatic lung cancer [15]. This concept is currently investigated in a prospectively randomized trial (SPLENDOUR; NCT02129699). International guidelines published by the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) recommend the immediate start of either a bisphosphonate or denosumab as soon as BM have been identified, to reduce SREs as well as occurrence or aggravation of pain [16,17].
While there are some data on incidence and outcome of BM and SREs in NSCLC [18,19], data on incidence, outcome and treatment patterns of BM and SREs in SCLC are scarce and controversial. A recently published large retrospective register-based study from Denmark reported a BM incidence of 16.7% in 5900 SCLC patients [19]. More than 50% of patients presenting with BM suffered from an SRE over time. BM predicted a poor prognosis, while prognosis for patients with BM plus SRE was even worse. This study did not report on the role of antiresorptive drugs in this patient population. A small but prospective analysis from Japan revealed a much higher BM incidence rate of 40% and a dramatically lower SRE incidence rate of 9% in patients with ED-SCLC [20]. In this trial, BM incidence was similar to stage IV NSCLC patients (n=124, 47% BM) reported in the same analysis. Interestingly, the authors reported a high discrepancy in the therapeutic use of ZA between ED-SCLC and stage IV NSCLC (7% vs. 40%). Moreover, this study suggested LDH, known as a prognostic factor in LD and ED-SCLC [21], as an independent predictor for BM development in SCLC patients.
Based on these data we initiated our retrospective study aiming at (1) describing the incidence of BM and SREs in SCLC in a Swiss patient cohort and (2) exploring treatment algorithm of SCLC patients with BM and SREs and (3) evaluating the clinical relevance of LDH as a predictor of BM in this patient population.
2. Materials and Methods
2.1 Study design
This is a retrospective observational study based on a cohort of consecutive patients selected by the histopathological diagnosis of SCLC at our hospital over a 10-year period. The study was approved by the responsible local Ethical committee (Ethikkommission Nordwest- und Zentralschweiz (EKNZ)). The last follow up was performed in October 2015.
2.2 Criteria of database generation and follow-up schedule
The detailed process of database setup and collection has been reported previously [22]. Patient characteristics, treatment delivery, response, outcome, toxicity and established prognostic parameters were evaluated by record review. The review followed each patient from first diagnosis until death or until data cut off (31 Oct. 2015). Patients who were lost to follow-up were excluded from the final analysis.
BM was defined as any suspicious lesion described in the general tumor assessment using computed tomography (CT), radionuclide bone scan, integrated positron emission tomography (PET)-CT or magnetic resonance imaging (MRI).
SRE was defined as any of the following: Pathologic fracture, spinal cord compression, radiation or surgery to the bone and/or HCM. Fractures were identified by the radiological report of the routine assessment. Hypercalcemia was defined as an albumin corrected serum calcium above 2.65 mmol/l.
2.3 Statistical analyses
Statistical analyses were performed using the Excel software program (version 2010). Analyses were performed for the entire patient cohort and percentages and descriptive statistics were used to summarize the data. To evaluate the role of LDH as a BM predicting factor, univariate logistic regression analysis was done. LDH was categorized in LDH ≤300/ >300 U/l, as well as in LDH ≤1000/ >1000 U/l. We used this cut off based on the publications by Hermes and Katakami et al. [20,21]. SPSS statistical software version 22 (IBM Corporation, New York, USA) was used.
3. Results
3.1 Patients
92 patients with histologically confirmed SCLC either limited (LD) (24%) or extensive disease (ED) (76%) were identified between January 2000 and December 2010 at our institution. As listed in Table 1, the median age of the patient population was 62.7 (range 39.7-81.9) years. There was a male predominance (67%). The ECOG performance status (PS) was 0-1 in 83% of patients. In the whole cohort, the median OS was 10.3 months (range 2.3-20.5). At the time of data cut off 83 patients were deceased and three patients were still alive. Six patients were lost to follow-up and were therefore not included in outcome analyses. At first diagnosis, 37% of all ED-SCLC patients presented with BM, while 8.7% presented with a SRE.
Baseline characteristics of SCLC patients at first diagnosis.
| LD | ED | Total | |
|---|---|---|---|
| N (%) | 22 (23.9) | 70 (76,1) | 92 (100) |
| Age (years) median [range] | 65.6 [47.4 - 78.0] | 61.3 [39.7 - 81.9] | 62.7 [39.7 - 81.9] |
| Sex (female/male) [%] | 6/16 [27.3/72.7] | 24/46 [34.3/65.7] | 30/62 [32.6/67.4] |
| PS (ECOG) | (missing 5) | (missing 29) | (missing 34) |
| 0 (%) | 5 (29.4) | 17 (41.5) | 22 (38) |
| 1 | 10 (58.8) | 16 (39) | 26 (45) |
| 2 | 1 (5.8) | 7 (17.1) | 8 (14) |
| ≥ 3 | 1 (5.8) | 1 (2.4) | 2 (4) |
| BM (%) | |||
| - | 22 (100) | 36 (51.4) | 58 (63.1) |
| + | 0 | 34 (48.6) | 34 (36.9) |
| SRE | |||
| - | 22 (100) | 62 (88.6) | 84 (91.3) |
| + | 0 | 8 (11.4) | 8 (8.7) |
| Chemotherapy | |||
| - | 0 | 1 (1.4) | 1 (1.08) |
| + | 22 (100) +/- radiotherapy | 69 (98.6) | 91 (98.92) |
| Bisphosphonates | |||
| - | 21 (95.5) | 53 (75.7) | 74 (80.4) |
| + | 1 (4.5) | 17 (24.3) | 18 (19.6) |
3.2 Treatment algorithm
All patients with LD-SCLC received chemotherapy with or without radiotherapy as initial treatment. In patients with ED-SCLC 98.6% received palliative chemotherapy. The majority of patients were treated with platinum and etoposide as first-line therapy. Overall, 19.6% received a bone targeted therapy with a bisphosphonate, either ZA (92%) or bondronate (8%). Denosumab was not licensed at the time of trial conduct. In LD-SCLC one patient was treated with ZA due to severe osteoporosis. In ED-SCLC only 50% (17/34) of patients received a bisphosphonate at first occurrence of BM.
3.3 Incidence of BM in LD and ED
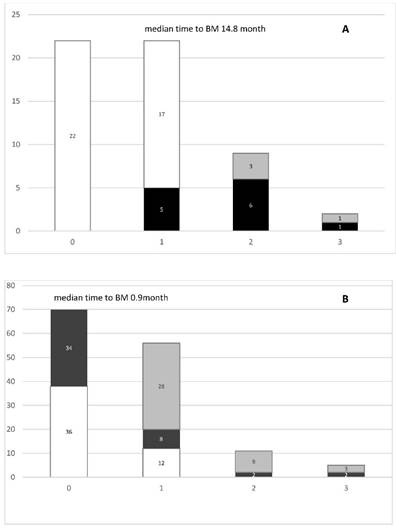
We analyzed the incidence of BM in both LD- and ED-SCLC patients (Figure 1). In LD-SCLC patients, 5 out of 22 (23%) patients developed BM at the time of first relapse. At second recurrence, 33% (3 patients of 9) showed stable BM and 67% new or progressive lesions. At third recurrence, BM were stable in 1 patient and 1 patient showed new BM. The median time to occurrence of BM in LD-SCLC patients was 14.8 months (range) (Figure 1A). In ED-SCLC patients, 48% (34 patients of 70) presented with BM at first diagnosis. At first recurrence, there were 25% (12 patients of 48) with no, 17% with new and 58% with stable BM. With regard to the 34 patients initially diagnosed with bone metastases, 82% (28 patients) showed stable disease in the bone at time of first recurrence. At second recurrence, 82% (9 of 11 patients) presented with stable and 18% (2 patients) with new BM. At third recurrence, 60% showed stable bone disease and 40% were diagnosed with new BM. Overall, in ED-SCLC the median time to BM development was 0.9 months (range) (Figure 1B).
A. Incidence of bone metastases (BM) in SCLC-LD patients (n=22). B. Incidence of BM in SCLC-ED patients (n=70). White bars= no BM, Black bars= new BM, Gray bars= stable BM.
3.4 Type and incidence of SRE in SCLC
At baseline, SREs were documented in 8 patients (8.7%). Total number of patients developing SRE at first, second or third recurrence was 8 (10.3%), 3 (15%) and 1 (16.6%), respectively. Overall, SRE occurred in 20 patients (18.4%). 18 of 20 (90%) SREs occurred in patients with known BM. The most frequent type of SRE was radiation to the bone with 10.9% (10 of 92 patients) (4.8% at baseline (4 of 92), 7.1% (5 of 78) at first recurrence and 5% (1 of 20) at second recurrence, respectively) (Table 2 and 4). Pathologic fracture, surgery to bone, spinal cord compression and HCM were rare events (overall 10.9%, 10 patients total) (Table 2 and 4). At baseline, 6 out of 8 (75%) patients with SRE received a bisphosphonate. Antiresorptive therapy was initiated in 100% of patients presenting a SRE at first, second and third recurrence (Table 2). Overall, 90% of patients presenting SREs received bisphosphonates (ZA only).
3.5 LDH as a predictive factor for BM in SCLC patients
We further analyzed the role of LDH in predicting BM in our patient cohort. We performed univariate logistic regression analysis in 85 patients. At baseline, LDH levels ranged from 105 to 4838 U/l with a median level of 435.8 U/l. We detected elevated LDH levels (LDH > 225 U/l) in 44% of all patients. LDH levels ≥ 300 U/l (OR 3.75 (1.46; 9.63), p=0.06) and ≥ 1000 U/l (OR 5.63 (1.02; 31.07), p=0.05), as well as age ≥75 years (OR 0.27 (0.10; 0.72), p=0.009) predicted occurrence of BM in LD-SCLC and ED-SCLC (Table 3).
Type and incidence of SRE in SCLC (all LD +ED).
| baseline (0) | first recurrence (1) | second recurrence (2) | third recurrence (3) | overall | |
|---|---|---|---|---|---|
| n | 92 | 78 | 20 | 6 | 92 |
| Pathologic fracture (%) | 1 (1.1) (1 new BM diagnosis) | 1 (1,3) (1 new BM diagnosis) | 1 (5) (known BM) | -- | 3 (3.3) |
| Radiotherapy to bone (%) | 4 (4.8) (4 new BM diagnosis) | 5 (7,1) (3 new BM diagnosis) | 1 (5) (1 new BM diagnosis) | -- | 10 (10.9) |
| Surgery to bone (%) | 1 (1.1) (known BM) | 1 (1,3) (known BM) | -- | -- | 2 (2.2) |
| Spinal cord compression (%) | 1 (1.1) (1 new BM diagnosis) | -- | -- | -- | 1 (1.2) |
| Hypercalcemia (%) | 1 (1.1) (1 new BM diagnosis) | 1 (1.7) (known BM) | 1 (5) (no BM diagnosis) | 1 (16.6) (no BM diagnosis) | 4 (4.3) |
| SRE total (%) SRE +BM | 8 (8.7) | 8 (10.3) | 3 (15) | 1 (16.6) | 20 (18.4) |
| 18 (90) | |||||
| Bisphosphonate treatment (% of SRE total) | 6 (75) | 8 (100) | 3 (100) | 1 (100) | 18 (90) |
Predictors of metastases (univariate logistic regression analysis).
| Outcome = BM | n | Odds Ratio (95%CI) |
|---|---|---|
| age≥75 | 91 | 0.27 (0.10; 0.72), p=0.009 |
| LDH≥300 | 85 | 3.75 (1.46; 9.63), p=0.06 |
| LDH≥1000 | 85 | 5.63 (1.02; 31.07), p=0.05 |
| Performance high (≥1) | 53 | 0.64 (0.20; 2.05), p=0.45 |
| Male sex | 91 | 1.05 (0.41; 2.64), p=0.93 |
| ED | 91 | 0.44 (0.16; 1.17), p=0.10 |
(BM= bone metastases, N= total number patients, ED=extensive disease).
4. Discussion
This is one of the first and most comprehensive reports on incidence rate and treatment strategies of BM and SRE in a Caucasian SCLC patient cohort. Overall, there was a 36.9% incidence of BM. Half of BM diagnosis occurred at first disease presentation (48.6%). In a recently published Japanese prospective trial, the incidence of BM in SCLC was reported to be 40.4%. In a recent publication from Denmark, the BM incidence was substantially lower (16.7%). Results of the three studies on BM and SRE in SCLC are summarized in Table 4. All three trials reported the years between 1999-2010 were denosumab was not yet available in the treatment of BM. Age and sex distributions were comparable reflecting that SCLC is mainly present in older patients (median age: 68 (Danish), 68 (Japanese) and 62 (Swiss) years, respectively), men (male patients 57%, 70%, 62%, respectively). There are various reasons for a different BM incidence. The Danish study encounters a large patient population (n=5900). The much smaller two other studies might reflect less robust data. Nevertheless, evaluations occurred with different methodology (cumulative incidence analysis versus descriptive statistics). Variability of BM incidences has also been described in NSCLC. According to several trials published in the 1990s, the mean incidence of BM in NSCLC is 20% [23,24] while more recent publications describe a higher incidence of 30% [25]. This might be due to the use of new imaging modalities, e.g. PET/CT and MRI scans with a higher BM detection rate [26,27]. While there is no description on how BM have been evaluated in the Danish population, Swiss and Japanese trials both reported BM evaluation by either CT, PET/CT and/or MRI scans during regular disease staging. It is only speculative, if PET/CT might have been less accessible by the time of study in Denmark [28] and therefore could reflect a lower BM detection rate in SCLC patients.
In LD SCLC, we observed a median time to develop BM from first diagnosis of 14.8 months, compared to 19 months in the Japanese cohort. The overall BM incidence in the larger cohort from Denmark was 5.1%/year. Based on this data, we strongly suggest screening for BM during the follow up of LD-SCLC patients after the initial treatment period.
The overall and baseline incidence of SREs in our patient cohort was 18.4% and 8.7%, respectively. The results are similar to the Japanese and Danish cohorts (overall incidence 18.2% and 14.8% respectively) (Table 4). Compared to the Danish population, where 50% of all BM patients presented a SRE, our Swiss population showed a higher SREs incidence of 90% in BM patients. Current recommendations strongly suggest the use of either bisphosphonates or denosumab in patients with BM to prevent SREs. The higher incidence of SREs in SCLC patients with BM in our retrospective study might reflect the “real world” complexity of these patients who, first, might not be able to receive efficient bone-targeted therapy, due to poor performance status (62% PS 1- ≥3 in our Swiss population). Second, patients might present a rapid clinical deterioration despite chemotherapeutic treatment, while waiting for a dental treatment before start of an antiresorptive drug. Finally, bisphosphonates might be less effective in preventing SREs in SCLC patients as compared to patients with other malignant diseases (e.g. breast or prostate cancer). Nevertheless, current evidence supports an early start of antiresorptive drugs to prevent SREs in patients with BM as the main role of bone-targeted therapies lays in primary prevention of SRE [6,9-11].
Our study showed that the most common SRE was radiation therapy to bone, followed by HCM, pathologic fractures, surgery to the bone and spinal cord compression. Taking NSCLC and SCLC data together, radiotherapy to the bone reflects the most common SRE, whereas the frequency of surgery to the bone seems to be higher in Western countries compared to Japan [19,25,29].
Overview of the literature on BM and SRE in SCLC.
| Denmark (Cetin K et al., 2014) | Japan (Katakami N et al., 2014) | Switzerland | |
|---|---|---|---|
| Trial Design | Retrospective, population-based | Prospective, observational | Retrospective, observational |
| duration | 1999-2010 | 2008-2009 | 2000-2010 |
| cancer type | NSCLC and SCLC | NSCLC and SCLC | SCLC |
| n | 5900 (SCLC only) | 77 (SCLC only) | 92 (SCLC only) |
| LD: n.n. | LD: 30 | LD:22 | |
| ED: n.n. | ED: 47 | ED:70 | |
| Median age | 68 years (15.7-104.3) | 68 years (35-89) | 62.7 [39.7 - 81.9] |
| Male sex | 57% | 70.4% | 62% |
| BM incidence | Overall: 16.7% | At first diagnosis: 40.4% | At first diagnosis: 36.9% |
| time to BM from first diagnosis | Overall: 5.1%/year | Overall: 19 months | LD: 14.8 months ED: 0.9 months |
| SRE incidence | Overall: 14.8% | Overall: 18.2% | Overall: 18.4% |
| 50% of all BM patients | -- | 90% of all BM patients | |
| At first diagnosis: -- | At first diagnosis: 8.5% | At first diagnosis: 8.7% | |
| SRE type | Overall (NSCLC+SCLC): | Overall (NSCLC + SCLC): | Overall (SCLC only) |
| Pathologic fracture: 8% | Pathologic fracture: 4.7% | Pathologic fracture: 3.3% | |
| Radiation to bone: 67% | Radiation to bone: 15.7% | Radiation to bone: 10.9% | |
| Surgery to bone: 4% | Surgery to bone: 0% | Surgery to bone: 2.2% | |
| Spinal cord compression: 21% | Spinal cord compression: 1.1% | Spinal cord compression: 1.2% | |
| HCM: -- | HCM: 2.2% | HCM: 4.3% | |
| time to SRE from first diagnosis | 48.2% per year | Overall: 9.5 month | -- |
| Bisphos-phonate use | -- | SCLC overall: 7.8% | SCLC overall: 19.6% |
| LD: 8.5% | LD: 4.5% | ||
| ED: 6.7% | ED: 24.3% | ||
| predictive factors for BM | -- | - ED (HR=6.11; 95% CI 1.69-22.05, p=0.006 | - age ≥ 75 (OR 0.27 (0.10; 0.72), p=0.009 |
| - LDH >1000 at baseline (HR=9.14; 95% CI 1.51-55.14, p=0.016 | - LDH ≥ 300 (OR 3.75 (1.46; 9.63), p=0.06 | ||
| - PTHrP elevation at baseline (HR=0.38; 95% CI, 0.15-0.99, p=0.048 | - LDH ≥ 1000 (OR 5.63 (1.02; 31.07), p=0.05 |
Both, the presence of BM as well as elevated serum LDH have been shown to be independent prognostic factors for SCLC survival outcome. The role of LDH as a predicting factor of BM occurrence in patients with SCLC has been suggested in one trial [20]. We confirmed this observation in our patient cohort and propose to assess the role of LDH in predicting BM in SCLC prospectively. Age was another predictor for BM in our SCLC cohort. As age is often associated with comorbidities, there is a tendency for less intensive treatment in older patients, a possible explanation for our finding [1]. Nevertheless, we suggest screening elderly patients suffering from SCLC for BM, especially in the palliative setting, as prevention of SREs and pain reduction are of great importance.
5. Conclusions
The incidence of BM in our retrospective SCLC cohort of 92 patients was 36.9%. Overall, 18.4% suffered a SRE. Only 19.6% of patients with BM received a bone-targeted treatment at baseline. The early use of bone-targeted treatments in SCLC patients presenting with BM to prevent SRE should be acknowledged. In SCLC patients, elevated serum LDH levels at baseline are a predictive factor for the development of BM. Based on these data we plan a prospective trial assessing the role of denosumab in preventing BM and SRE in SCLC patients with elevated LDH.
Acknowledgements
The authors thank Mrs. Stefanie Aeschbacher for statistical assistance and Dr. David Conen for proof reading the article.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Only the authors had access to the data used. Analysis, presentation and interpretation of the results are solely the responsibility of the authors.
References
1. Stupp R, Monnerat C, Turrisi AT, Perry MC, Leyvraz S. Small cell lung cancer: state of the art and future perspectives. Lung Cancer. 2004;45:105-17 doi:10.1016/j.lungcan.2003.12.006
2. Mountain CF. The international system for staging lung cancer. Semin Surg Oncol. 2000;18:106-15
3. Carney DN. Lung cancer-time to move on from chemotherapy. N Engl J Med. 2002;346:126-8 doi:10.1056/NEJM200201103460211
4. Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J. et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125-32 doi:10.1200/JCO.2010.31.3304
5. Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C. et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785-91 doi:10.1056/NEJM199612123352401
6. Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M. et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial-the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150-7 doi:10.1200/JCO.2003.04.105
7. Bienz M, Saad F. Management of bone metastases in prostate cancer: a review. Curr Opin Support Palliat Care. 2015;9:261-7 doi:10.1097/SPC.0000000000000157
8. De Castro J, García R, Garrido P, Isla D, Massuti B, Blanca B. et al. Therapeutic Potential of Denosumab in Patients With Lung Cancer: Beyond Prevention of Skeletal Complications. Clin Lung Cancer. 2015;16:431-46 doi:10.1016/j.cllc.2015.06.004
9. Hirsh V, Major PP, Lipton A, Cook RJ, Langer CJ, Smith MR. et al. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol. 2008;3:228-36 doi:10.1097/JTO.0b013e3181651c0e
10. Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M. et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613-21 doi:10.1002/cncr.20308
11. Hirsh V, Tchekmedyian NS, Rosen LS, Zheng M, Hei Y-J. Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clin Lung Cancer. 2004;6:170-4 doi:10.3816/CLC.2004.n.030
12. Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst Rev. 2002 CD002068. doi:10.1002/14651858.CD002068
13. Stopeck AT, Lipton A, Body J-J, Steger GG, Tonkin K, de Boer RH. et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132-9 doi:10.1200/JCO.2010.29.7101
14. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet (London, England). 2011;377:813-22 doi:10.1016/S0140-6736(10)62344-6
15. Scagliotti GV, Hirsh V, Siena S, Henry DH, Woll PJ, Manegold C. et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012;7:1823-9 doi:10.1097/JTO.0b013e31826aec2b
16. Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS. et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221-7 doi:10.1200/JCO.2010.32.5209
17. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25(Suppl 3):iii124-37 doi:10.1093/annonc/mdu103
18. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588-94
19. Cetin K, Christiansen CF, Jacobsen JB, Nørgaard M, Sørensen HT. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86:247-54 doi:10.1016/j.lungcan.2014.08.022
20. Katakami N, Kunikane H, Takeda K, Takayama K, Sawa T, Saito H. et al. Prospective study on the incidence of bone metastasis (BM) and skeletal-related events (SREs) in patients (pts) with stage IIIB and IV lung cancer-CSP-HOR 13. J Thorac Oncol. 2014;9:231-8 doi:10.1097/JTO.0000000000000051
21. Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer - a retrospective single institution analysis. Respir Med. 2010;104:1937-42 doi:10.1016/j.rmed.2010.07.013
22. Hagmann R, Hess V, Zippelius A, Rothschild SI. Second-Line Therapy of Small-Cell Lung Cancer: Topotecan Compared to a Combination Treatment with Adriamycin, Cyclophosphamide And Vincristine (ACO) - a Single Center Experience. J Cancer. 2015;6:1148-54 doi:10.7150/jca.13080
23. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-76 doi:10.1053/ctrv.2000.0210
24. Kosteva J, Langer C. The changing landscape of the medical management of skeletal metastases in nonsmall cell lung cancer. Curr Opin Oncol. 2008;20:155-61 doi:10.1097/CCO.0b013e3282f54cf2
25. Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229-32 doi:10.1016/j.lungcan.2007.03.013
26. Schumacher T, Brink I, Mix M, Reinhardt M, Herget G, Digel W. et al. FDG-PET imaging for the staging and follow-up of small cell lung cancer. Eur J Nucl Med. 2001;28:483-8
27. Cheran SK, Herndon JE, Patz EF. Comparison of whole-body FDG-PET to bone scan for detection of bone metastases in patients with a new diagnosis of lung cancer. Lung Cancer. 2004;44:317-25 doi:10.1016/j.lungcan.2003.11.008
28. Petersen H, Holdgaard PC, Madsen PH, Knudsen LM, Gad D, Gravergaard AE. et al. FDG PET/CT in cancer: comparison of actual use with literature-based recommendations. Eur J Nucl Med Mol Imaging. 2015 doi:10.1007/s00259-015-3217-0
29. Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J. et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390-6 doi:10.1159/000082923
Author contact
![]() Corresponding author: Katrin Conen, MD, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland, katrin.conench
Corresponding author: Katrin Conen, MD, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland, katrin.conench

 Global reach, higher impact
Global reach, higher impact