3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2016; 7(10):1215-1225. doi:10.7150/jca.15395 This issue Cite
Research Paper
Clinical Significance of “Double-hit” and “Double-protein” expression in Primary Gastric B-cell Lymphomas
1. Department of Pathology, Changhai Hospital, The Second Military Medical University, Shanghai 200433, CHINA
2. Molecular Pathology, Cellular & Molecular Pathology Branch, National Institutes of Health, Research Triangle Park, NC 27709, USA
3. Department of Hematology, Changhai Hospital, The Second Military Medical University, University, Shanghai 200433, CHINA
4. Department of Pathology, Children Hospital and Women Health Center of Shanxi, Taiyuan 030013, CHINA
5. Division of Molecular Pathology, Joint Pathology Center, Washington, DC 20817, USA.
*These authors contributed equally to this work.
Received 2016-2-27; Accepted 2016-4-26; Published 2016-6-18
Abstract
BACKGROUND AND AIMS: Primary gastric B-cell lymphoma is the second most common malignancy of the stomach. There are many controversial issues about its diagnosis, treatment and clinical management. “Double-hit” and “double-protein” involving gene rearrangement and protein expression of c-Myc and bcl2/bcl6 are the most used terms to describe DLBCL poor prognostic factors in recent years. However, very little is known about the role of these prognostic factors in primary gastric B-cell lymphomas. This study aims to obtain a molecular pathology prognostic model of gastric B-cell lymphoma for clinical stratified management by evaluating how the “double-hit” and “double-protein” in tumor cells as well as microenvironmental reaction of tumor stromal tissue affect clinical outcome in primary gastric B-cell lymphomas.
METHODS: Data and tissues of 188 cases diagnosed with gastric B-cell lymphomas were used in this study. Tumor tissue microarray (TMA) of formalin fixed and paraffin embedded (FFPE) tissues was constructed for fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) analysis with a serial of biomarkers containing MYC, BCL2, BCL6, CD31, SPARC, CD10, MUM1 and Ki-67. Modeled period analysis was used to estimate 3-year and 5-year overall survival (OS) and disease-free survival (DFS) distributions.
RESULTS: There was no definite “double-hit” case though the gene rearrangement of c-Myc (5.9%), bcl2 (0.1%) and bcl6 (7.4%) was found in gastric B-cell lymphomas. The gene amplification or copy gains of c-Myc (10.1%), bcl-2 (17.0%) and bcl-6 (0.9%) were present in these lymphomas. There were 12 cases of the lymphomas with the “double-protein” expression of MYC and BCL2/BCL6. All patients with “double-protein” gastric B-cell lymphomas had poor outcome compared with those without. More importantly, “MYC-BCL2-BCL6” negative group of gastric B-cell lymphoma patients had favorable clinical outcome regardless clinical stage, pathological types and therapeutic modalities. And the similar better prognosis was found in the cases with low microvessel density (MVD) in tumor tissue and high expression of SPARC (SPARC≥5%) in stromal cells.
CONCLUSIONS: “Double-hit” lymphoma was rare among primary gastric lymphoma, while patients with multiple gene amplification and/or copy gains of c-Myc, bcl2 and bcl6, and “double-protein” gastric B-cell lymphomas had a poor clinical outcome. In addition, patients with MYC, BCL2 and BCL6 expression negative or low MVD in tumor tissue with high expression of SPARC in stromal cells could have better prognosis than other gastric B-cell lymphomas regardless of their clinical stage and pathological types. These results would be of very importance for clinical stratified management and precision medicine of gastric B-cell lymphomas.
Keywords: Primary gastric B-cell lymphoma, Double-hit, Double-protein, MVD, SPARC, Fluorescent in situ hybridization, Immunohistochemistry and Tumor tissue microarray.
Introduction
Primary gastric lymphoma represents nearly 30-45% of extranodal lymphomas, which is a member of the family of neoplasm mainly derived from B-cells [1]. Gastric B-cell lymphoma is also the second most common malignancy of the stomach, which consists of two predominant histological subtypes, accounting for around 90% of all gastric lymphomas [2].The low-grade subtype is called extranodal marginal zone lymphoma of mucosal-associated lymphoid tissue (MALT), while the high-grade subtype is mainly diagnosed as diffuse large B-cell lymphoma (DLBCL) [2,3]. Though gastric MALT lymphoma is strongly associated with Helicobacter pylori (H. Pylori) infection, a proportion of MALT lymphoma patients are H. Pylori negative or are diagnosed at advanced stage, in whom antibiotics treatment may not always be effective [4,5]. In contrast to MALT lymphoma, etiological factors for DLBCL are not well established [1].The role of H. Pylori is still controversial in the etiology of DLBCL of the stomach. Whether DLBCL arises de novo in the stomach or they transform from low grade MALT lymphomas, is unclear [1,4,5]. Despite the ability of gastric lymphomas to spread or transform, most cases remain localized within the stomach for many years [6]. In recent years, the incidence of primary gastric lymphomas is getting higher and the prognosis of the gastric lymphoma is better than gastric carcinoma [2,3]. Historically, surgical intervention played a critical role in the multimodal management of gastric lymphoma [6,7]. However, with the emergence of data demonstrating comparable outcomes with lower morbidity and better quality of life with chemotherapy and radiation, there has been a shift toward non-operative management for these patients [8,9]. In fact, there is still no consensus on the optimal treatment for gastric B-cell lymphoma, especially for H. pylori negative MALT lymphomas, and more aggressive treatment is also commonly used for gastric DLBCL in clinical setting. Meanwhile, the prognosis of gastric lymphoma has been based largely on clinical factors. The same clinical factors could not explain why patients at the same stage of the disease and with similar clinical characteristics have different prognoses. Therefore, these factors cannot be used to safely determine which patients could benefit from operation, adjuvant chemotherapy or radiotherapy [2,3].
Nowadays, our understanding of the pathogenesis and heterogeneity of DLBCL has been dramatically enhanced by recent attempts to profile molecular features of the malignancy [10]. In particular, the recent insight into the molecular heterogeneity of B-cell lymphoma, especially DLBCL, is driving a paradigm shift toward more personalized evaluation and therapies [10,11]. The most used term to refer to DLBCL is the “double-hit” lymphomas in recent years, with c-Myc and bcl2 and/or bcl6 rearrangements identified based on cytogenetic testing [11]. Also, there is growing interest in the more common “double-protein” lymphomas, which is defined as co-expression of two oncogenes (MYC and BCL2 and/or BCL6) on more than a specified proportion of tumor cells based on immunohistochemical staining [11,12]. Most “double-protein” lymphomas that are not “double-hit” are of activated B-cell origin. Several groups have validated the “double-protein” status as a viable biomarker in R-CHOP treated patients [10-12]. MYC/BCL2 co-expression, rather than cell-of-origin classification, is the best predictor of prognosis in patients with DLBCL treated with R-CHOP because the majority of treatment failures after R-CHOP are in “double-protein” DLBCL [13]. How do the “double-hit” and “double-protein” relate to the patients with primary gastric B-cell lymphomas? There are few reports about the combination of abnormalities of MYC and BCL2 or BCL6 in gastric B-cell lymphomas [1-3].
On the other hand, although the microenvironment has long been recognized as an important participant in the pathogenesis of tumors, the nature of this relationship is still not well understood [14,15]. Cells in the microenvironment may support tumor cell growth and survival or may be part of a host response against the tumor [14]. Morphologically, gastric B-cell lymphomas always have prominent stromal reactive inflammatory cells such as macrophages, plasma cells, eosinophils and so on [1]. Gastric MALT lymphoma has been proposed to be a model of chronic inflammation induced tumor development [16]. Alternative strategies of organizing gene expression data emphasize heterogeneity of DLBCL with subsets characterized by signatures of host response [14-16]. Lenz et al showed increased survival for patients with DLBCL in which the tumor microenvironment expressed a group of genes referred to as the “stromal-1” signature [17]. SPARC (secreted protein, acidic and rich in cysteine), is present on the list of stromal-1 signature genes. Meyer et al thought SPARC was associated with increased DLBCL patients' survival, presumably owing to tumor suppression [18]. Cardesa-Salzmann et al observed that high microvessel density determined a poor outcome in patients with DLBCL treated with rituximab plus chemotherapy [19]. To this regard, the tumor microenvironment of gastric B-cell lymphomas might be essential and need further investigation.
The aim of this study is to better define molecular and clinical features of gastric B-cell lymphomas. We have focused on comparing serial biological markers related to the “double-hit” and “double-protein” in tumor cells, SPARC expression in stromal cells and tumor angiogenesis in patients with primary gastric B-cell lymphomas using immunohistochemical and molecular pathological methods. Combining clinic-pathological characteristics, treatment and prognosis analysis of these patients, we found that “double- protein” expression were not “double-hit” lymphoma, and high expression SPARC in stromal cells indicate better prognosis of gastric B-cell lymphoma, which, we hope, will become a molecular pathology prognostic model for clinical stratified management of this malignancy and for implementation of precision medicine in the field.
Material and Methods
Patients
Pretreatment tumor-biopsy or surgical specimens and clinical data were obtained from 230 patients with newly diagnosed gastric B-cell lymphomas at Department of pathology, Changhai Hospital, the Second Military Medical University, from 2001 through 2013 according to a protocol approved by the institutional review board of the Changhai Hospital. A panel of expert hemato-pathologists confirmed the diagnosis of B-cell lymphoma using current World Health Organization classification criteria [1]. A total of 188 out of 230 patients were treated in Changhai Hospital and approval from the local ethics committee and informed consent of the patients or their relatives were obtained prior to recruit into the study. Retrospective review included patient demographics, medical history, preoperative pathology, nodal status, pathologic characteristics and bone marrow biopsy. Lactate dehydrogenase (LDH) and β2-microglobulin were considered high at 310U/L and 2.8 mg/L, respectively. Patients were staged according to the Lugano International Conference classification (I, II II2, II E and IV), treatment with chemotherapy and/or radiation, operation reports, clinic visits, and recurrence events. Eastern Cooperative Oncology Group score (ECOG) was also used for the prognosis analysis. Patients in the study were divided into 3 prognostic groups according to IPI scores: low risk (0-I), intermediate risk (II-III), and high risk (IV-V).
Tissue microarray
Hematoxylin and eosin (H&E) stained sections from a representative FFPE tissue blocks were used to define diagnostic tumor areas, and 1 to 3 representative tissue cores, 0.6-1.0 mm in diameter, were obtained from each case and inserted into recipient paraffin blocks in a grid pattern using a tissue microarrayer (Beecher Instruments). Serial 4 - 5-μm sections of each TMA were stained with H&E and used for additional immunohistochemistry and FISH analysis.
Fluorescence In-Situ Hybridization (FISH)
Dual color break-apart FISH probes for c-Myc, bcl2 and bcl6, and dual color, dual fusion-translocation probe t(14;18)(q32q21) IgH/bcl2 were labeled with Spectrum Orange and Spectrum Green fluorescence (Abbott Molecular, Des Plaines, IL, USA). FISH assays were performed as described in literature [20]. Briefly, representative 4-5μm tissue sections on positively charged glass slides from each TMA block were deparaffinized with Hemo-D, rehydrated in graded ethanol, pretreated at 97°C and digested with pepsin, and hybridized with FISH probes at 37°C overnight. After wash, the slides were ounter-stained with DAPI, and stored at -20°C. FISH images were scanned and analyzed using the Duet BioView image system (BioView, Boston, MA, USA). At least 100 nuclei from each sample were scored. FISH signal pattern of break-apart probes in normal cells presents two yellow signals or closely positioned red (organ color on DAPI purple background shows red color) and green signal(s) with a distance <2 signal sizes apart. A cell with one yellow, one green and one red signals with a distance ≥2 signal sizes has been defined as positive for gene rearrangement. In FISH assays with the dual-color, dual-fusion t(14;18)(q32q21) IgH/bcl2 probe, a cell without translocation typically has two separate green signals and two separate red signals. Some artificial fusion signals (false-positive) could be seen in cells, owing to overlays of green and red signals. The cut-off levels of 5% and 17% were established for break-apart gene rearrangement probes and t(14;18) IgH/bcl2 fusion-translocation probe, respective. A case with positive cells greater than the cut-off levels was defined as positive.
Immunohistochemistry (IHC)
One representative block from each case was stained with a minimal immunohistochemical panel including antibodies against Ki-67, MYC, BCL2, BCL6, CD10, MUM1, CD31 and SPARC. All antibodies were obtained from Dako (Carpinteria, CA, USA) and the immunohistochemical staining was performed according to the manufacturer's instructions. The immunoperoxidase staining, except for CD31, were done on Bond Max immunostainer (Vision BioSystems). The score of these antibody staining was estimated visually in 10% increments by 2 pathologists. The cut-off of Ki-67 and MYC were estimated by 40%. The number of stromal cells and histocytes in the tumor microenvironment that expressed SPARC was also estimated by 2 pathologists in each case and graded in 5% increments. The number of positive cells ranged from 0%-30%, and two groups of SPARC score were defined: <5% and ≥5% based on reports of previous studies [17,18]. The agreement between the 2 scoring authors was 90%. Any discrepancies were resolved by joint review over a double-headed microscope.
Blood vessel density measurement
Immunoperoxidase staining of CD31 was performed according to a previously described method [19]. The CD31 stains were performed for microvessel density (MVD) analysis on an automated Bond Max immunostainer (Vision BioSystems). The MVD was quantified by analyzing digitalized images of the CD31-stained tissue microarray cores with Olympus Cell Basic Imaging Software. Microvessel areas were defined as vascular areas delineated by CD31-positive staining, and the MVD was calculated as the sum of all microvessel areas (mm2) divided by the total area of the core analyzed (mm2). The MVD values were then grouped into quartiles (First quartile:0-0.00635; Second quartile: 0.00635-0.01047; Third quartile: 0.01047-0.01509; Fourth quartile: 0.01509-0.08270) and then get the final percentage [19]. The cut-off of CD31 percentage was estimated by 10%. The TMAs were independently scored by 2 observers, and discrepancies were resolved by joint review over a double-headed microscope.
Treatment modality
The patients were treated with four therapeutic modalities: surgery, chemotherapy, H. pylori eradication and radiotherapy. The purpose of surgery was to remove the tumor tissue and to treat complications, such as perforation and intractable bleeding. Chemotherapy included 6, 8, 21-day cycles with the CHOP [cyclophosphamide (750 mg/m2, day 1), doxorubicin (50 mg/m2, day 1), vincristine (1.4 mg/m2, day 1) and prednisone (100 mg, days 1-5)] or R-CHOP [rituximab (350 mg/m2, day1), cyclophosphamide (750 mg/m2, day1), doxorubicin (50 mg/m2 , day 1), vincristine (1.4 mg/m2, day1) and prednisone (100 mg, days 1- 5)] regimens. The H. Pylori eradication regimen consisted of proton pump inhibitors [omeprazole (20 mg, twice daily), lansoprazole (30 mg, twice daily) or rabeprazole (10 mg, twice daily)] and a combination of antibiotics [amoxicillin (1,000mg, twice daily), clarithromycin (500mg, twice daily) and/or metronidazole (200 mg, twice daily)].
Statistical analysis
The Kaplan-Meier method was used to estimate overall survival (OS) and disease-free survival (DFS) distributions. OS was defined as the time from initial diagnosis to death from any cause or last contact. DFS was defined as the time until disease recurrence, progression, or death from disease or chemotherapy related toxicity, or last contact. Patients who were alive and relapse-free at the last follow-up were treated as censored. The log-rank test was used to compare the survival distributions. The effects of clinical variables on DFS and OS were assessed with univariate analysis. The Cox proportional hazards regression model was used for the multivariate analysis. All analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) software. The statistical level of significance was defined as P<0.05.
Results
Clinical and pathological characteristics
Data of 188 patients with gastric B-cell lymphomas were total retrospectively analyzed. The antrum of the stomach was the predominant site of these lesions. Dyspepsia were the main symptoms at presentation in 70% of patients, followed by stomachache, nausea and vomiting in 63% of patients, and weight loss, fever, or night sweats (B symptoms) in 30% patients. The median age at presentation was 51 years (range: 17-85 years). 92 patients (49%) were male and 96 patients (51%) were female. The histological subtypes of the gastric B-cell lymphomas in this study were 32 (17%) cases of MALT lymphomas, 27(14%) case of DLBCL with MALT and129 (69%) cases of DLBCL (Fig. 1A, 1B). Of the188 total patients, 120 (64%) was ECOG low grade and 68 (36%) was ECOG high grade. 157(83.5%) had stage I-II and 31 (16.5%) had stage IIE-IV disease. 103(55%) was low risk, 62(33%) was intermediate risk and 23 (12%) was high risk according to IPI score. H. Pylori infection, B symptoms, stage, ECOG score, IPI score and treatment had statistical significance. The clinical characteristics of the patients are shown in Table 1.
Clinical and histopathological characteristics of the 188 cases of gastric B-cell lymphoma used in this study.
| Characteristics/ Outcomes | Number(%) patients | |||
|---|---|---|---|---|
| MALT-BCL (n=32) | DLBCL - MALT (n=27) | DLBCL(n=129) | P Value** | |
| Age (Years) | ||||
| < 60 | 20/32(62.5%) | 15/27(55.6%) | 77/129(59.7%) | 0.863 |
| ≥ 60 | 12/32(37.5%) | 12/27(44.4%) | 52/129(40.3%) | |
| Gender | ||||
| Male | 16/32(50.0%) | 17/27(63%) | 59/129(45.7%) | 0.265 |
| Female | 16/32(50.0%) | 10/27(37%) | 70/129(54.3%) | |
| H. pylori | ||||
| Negative | 7/32(21.9%) | 10/27(37%) | 91/129(70.5%) | <0.001 |
| Positive | 25/32(78.1%) | 17/27(63%) | 38/129(29.5%) | |
| β2-MG level | ||||
| Normal | 17/24(70.8%) | 12/17(70.6%) | 77/97(79.4%) | 0.548 |
| High | 7/24(29.2%) | 5/17(29.4%) | 20/97(20.6%) | |
| LDH level | ||||
| Normal | 30/32(93.8%) | 21/26(80.8%) | 97/128(75.8%) | 0.787 |
| High | 2/32(6.2%) | 5/26(19.2%) | 31/128(24.2%) | |
| Ascites | ||||
| No | 31/32(96.9%) | 24/26(92.3%) | 118/129(91.5%) | 0.584 |
| Yes | 1/32(3.1%) | 2/26(7.7%) | 11/129(8.5%) | |
| B Symptoms | ||||
| No | 29/32(90.6%) | 21/27(77.8%) | 82/129(63.6%) | 0.008 |
| Yes | 3/32(9.4%) | 6/27(22.2%) | 47/129(36.4%) | |
| Mass | ||||
| No | 31/32(96.9%) | 25/27(92.6%) | 109/129(84.5%) | 0.115 |
| Yes | 1/32(3.1%) | 2/27(7.4%) | 20/129(15.5%) | |
| Stage | ||||
| I - II2 | 26/32(81.3%) | 22/27(81.5%) | 109/129(84.5%) | 0.003 |
| IIE - IV | 6/32(18.7%) | 5/27(18.5%) | 20/129(15.5%) | |
| ECOG score | ||||
| < 2 | 27/32(84.4%) | 18/27(66.7%) | 75/129(58.1%) | 0.021 |
| ≥ 2 | 5/32(15.6%) | 9/27(33.3%) | 54/129(41.9%) | |
| IPI Score | ||||
| 0 - I | 25/32(78.1%) | 20/27(74.1%) | 58/129(45%) | 0.001 |
| II - III | 5/32(15.6%) | 4/27(14.8%) | 53/129(41.1%) | |
| IV - V | 2/32(6.3%) | 3/27(11.1%) | 18/129(14%) | |
| Treatment | ||||
| Surgery | 1/32(3.1%) | 3/27(11.1%) | 8/129(6.2%) | 0.009 |
| CT | 10/32(31.3%) | 4/27(14.8%) | 18/129(14%) | |
| CT + Surgery | 8/32(25%) | 16/27(59.3%) | 36/129(27.9%) | |
| CT + Rituximab | 5/32(15.6%) | 2/27(7.4%) | 28/129(21.7%) | |
| CT + Surgery + Rituximab | 8/32(25%) | 2/27(7.4%) | 39/129(30.2%) | |
| OS (DFS) | ||||
| 3 years (%) | 87.5 (87.5) | 74.1 (74.1) | 75.6 (70.5) | |
| 5 years (%) | 87.5 (87.5) | 70 (70) | 66.6 (64) | |
* MALT Mucosa-associated lymph tissue B-cell lymphoma; DLBCL Diffuse large B-cell lymphoma; LDH Lactate dehydrogenase; β2-MG β2-microglobulin; OS Overall survival; IPI: International Prognostic Index; ECOG: Estern Cooperative Oncology Group score; DFS: Disease-free Survival; ** Chi Square test.
Gene rearrangement and copy number changes of c-Myc, bcl2 and bcl6
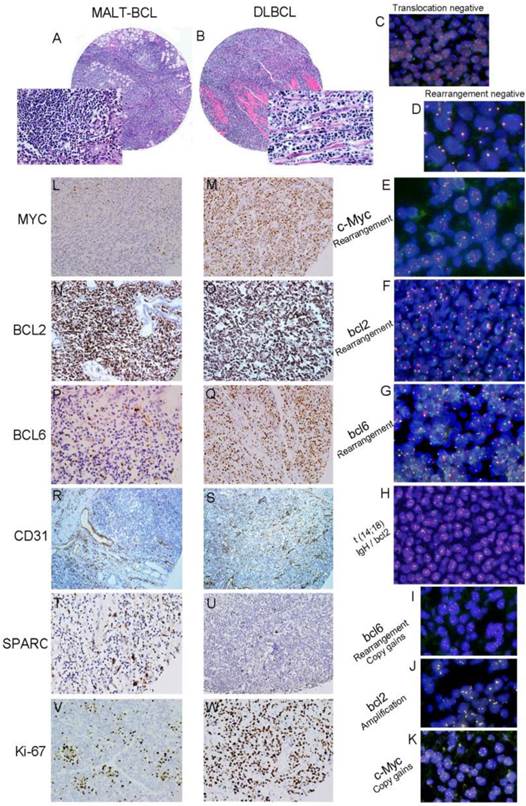
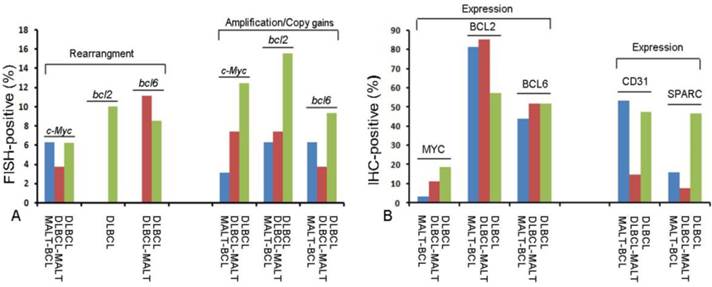
FISH assay was used to detect (14; 18) IgH/bcl2 translocation, the rearrangement, and copy number of c-Myc, bcl-2 and bcl-6 genes. The gene rearrangement were found in 11 cases (5.8%) for c-Myc, 1 case (0.6%) for bcl2 and 14 Cases (7.4%) for bcl6 in a total of 188 cases studied (Fig.1C-1G). There was only one case positive for t (14; 18) IgH/bcl2 translocation in these gastric B-cell lymphomas, which was DLBCL (Fig.1H). However, some cases were showed gene amplification or copy gains (also referred as copy number increase) of c-Myc of 19/188(10.1%), bcl-2 of 32/188 (17.0%) and bcl-6 of 17/188 (0.9%) respectively. Most of these cases were diagnosed as DLBCL (Fig.1I-1K) (Fig.2A; Table 2). Although the positive cases was too few, it was very interesting that 9 cases with double changes (either rearrangement or amplification/copy gains) of c-Myc and bcl-2 or bcl-6 were died of DLBCL during the following-up period. Two DLBCL patients with triple changes (either rearrangement or amplification/copy gains) of these three genes died even much earlier, within the first year after diagnosis regardless of their treatment modality.
Expression of serial biomarkers in the 188 cases of gastric B-cell lymphomas with histopathological data.
| Biomarkers | Number(%) patients | ||||
|---|---|---|---|---|---|
| MALT-BCL | DLBCL - MALT | DLBCL | P Value** | ||
| n=32 | n=27 | n=129 | |||
| c-Myc | |||||
| Rearrangement | 2(6.3%) | 1(3.7%) | 8(6.2%) | 0.559 | |
| Copy plus | 1(3.1%) | 2(7.4%) | 16(12.4%) | ||
| Expression | 1(3.1%) | 3(11.1%) | 24(18.6%) | 0.094 | |
| BCL-2 | |||||
| Rearrangement | 0 | 0 | 1(0.1%) | 0.369 | |
| Copy plus | 2(6.3%) | 2(7.4%) | 20(15.5%) | ||
| Translocation | 0 | 0 | 1(0.1%) | ||
| Expression | 26(81.3%) | 23(85.2%) | 74(57.4%) | 0.003 | |
| BCL-6 | |||||
| Rearrangement | 0 | 3(11.1%) | 11(8.5%) | 0.345 | |
| Copy plus | 2(6.3%) | 1(3.7%) | 12(9.3%) | ||
| Expression | 14(43.8%) | 14(51.9%) | 67(51.9%) | 0.703 | |
| CD10 | |||||
| Negative | 25(78.1%) | 17(63.0%) | 78(60.5%) | 0.178 | |
| Positive | 7(21.9%) | 10(37.0%) | 51(39.5%) | ||
| MUM1 | |||||
| Negative | 19(59.4%) | 11(40.7%) | 65(50.4%) | 0.363 | |
| Positive | 13(40.6%) | 16(59.3%) | 64(49.6%) | ||
| Ki-67 | |||||
| < 40 % | 32(100%) | 2(7.4%) | 2(1.6%) | <0.001 | |
| ≥40% | 0 | 25(92.6%) | 127(98.4%) | ||
| CD31 | |||||
| < 10 % | 17(53.1%) | 4(14.8%) | 61(47.3%) | 0.004 | |
| ≥10% | 15(46.9%) | 23(85.2%) | 68(52.7%) | ||
| SPARC | |||||
| < 5 % | 27(84.4%) | 25(92.6%) | 69(53.5%) | <0.001 | |
| ≥5% | 5(15.6%) | 2(7.4%) | 60(46.5%) | ||
* MALT: Mucosa-associated lymph tissue B-cell lymphoma;
DLBCL: Diffuse large B-cell lymphoma;
SPARC: Secreted protein, acidic and rich in cysteine.
** Chi square test.
Tissue microarray-based Fluorescence In Situ Hybridization (FISH) and immunochemistry (IHC) analysis of c-Myc, bcl2 and bcl6 gene rearrangement and protein expression in serial sections of gastric B-cell lymphoma specimens. Hematoxylin and eosin (H&E) staining of MALT lymphoma (A) and DLBCL (B) (TMA: HE×100, Inserted: HE×400.) Negative controls for t (14;18) IgH/bcl2 translocation (C) and gene rearrangement (D) (FISH ×1000). c-Myc (E: gene rearrangement with extra signals), bcl2 (F) and bcl6 (G) gene rearrangement were found in some DLBCLs and t (14; 18) IgH/bcl2 translocation was seen in one case of DLBCL (H). The amplification or copy gains of bcl6 (I: gene rearrangement with copy gains), bcl2 (J: gene amplication) and c-Myc (K: gene copy gains) (FISH ×1000) were observed in some gastric B-cell lymphomas. After immunostaining, a weak signal for MYC was seen in MALT lymphoma (L) and strong signal in DLBCL tumor cells (M). A stronger signal for BCL2 was seen both in MALT lymphoma (N) and DLBCL tumor cells (O). A weak signal for BCL6 was seen in MALT lymphoma (P) and strong signal in DLBCL tumor cells (Q). Low percentage for CD31 was seen in MALT lymphoma (R) and high percentage in DLBCL tumor cells (S). MALT lymphoma had high percentage staining for SPARC (M) in stromal cells; while low percentage staining for SPARC was seen in DLBCL stromal cells (N).Low percentage for Ki-67 was seen in MALT lymphoma (V) and high percentage in DLBCL tumor cells (W). (Brown colored cells are positive signal. IHC×200.)
Marker Protein expression
The protein expression of biomarker panel was measured by immunohistochemistry as described in Materials and Methods. The validation set included 188 patients with complete immunohistochemically data. MYC was positive in one out of 32 MALT cases (3.1%), three out of 27 cases of DLBCL with MALT lymphoma (11.1%) and 24 out of 29 DLBCL cases (18.6%) (Fig.1L 1M). BCL-2 was expressed in 81.3% of MALT lymphoma (26/32), 85.2% of DLBCL with MALT lymphoma (23/27) and 57.4%of DLBCL (74/129) (Fig.1N 1O). BCL-6 was expressed in 43.8% of MALT lymphoma (14/32), 51.9% of DLBCL with MALT lymphoma (14/27) and 51.9% of DLBCL (14/27) (Fig.1P 1Q) (Fig. 2B; Table 2). Only BCL2 expressions in different histological types were statistically significant. MYC and BCL-2 or BCL-6 co-expression (n=12) were significantly associated with poor clinical outcome and short OS (Fig. 3A 3B). The negative MYC, BCL2 and BCL6 (MYC-BCL2-BCL6-) phenotype (n=30) has been associated with a 100% 5-year survival rate, which is significantly different from other phenotypes in any above described pathological types (Fig.3B 3C). The OS of “double-protein” group and “MYC-BCL2-BCL6” negative group has also statistical significance (p=0.014) (Fig.3B).
Profile of molecular biomarkers in different pathological types of gastric B-cell lymphomas.(A) Fluorescence In Situ Hybridization (FISH) results for c-Myc, bcl2 and bcl6 gene changes; (B) Immunohistochemistry (IHC) results for MYC, BCL2, BCL6, CD31 and SPARC protein expression in different pathological types of gastric B-cell lymphomas.
Immunohistochemistry (IHC) and impact of MYC, BCL2, BCL6, CD31 and SPARC expression on the prognosis of gastric B-cell lymphomas patients. The expression of MYC and BCL2 or BCL6 (double-protein expression) was associated with low overall survival (OS, poor prognosis) in patients with gastric B-cell lymphomas. A: Compare “double-protein” negative “MYC-BCL2-/BCL6-” with “double-protein” positive “MYC+BCL2+/BCL6+” patients P=0.015; B: Compare triple- protein negative “MYC-BCL2-BCL6-” with double-protein positive “MYC+BCL2+/BCL6+” patients P=0.014; (C) Compare triple-protein negative “MYC-BCL2-BCL6-” with single- protein positive “MYC+/BCL2+/BCL6+” patients P=0.001, Overall survival (OS) was associated with “MYC-BCL2-BCL6-” negative in a better prognosis than other gastric B-cell lymphomas patients P=0.001). (D) Patients with low MVD (CD31<10%) and high SPARC positive in stromal cells (SPARC≥5%) showed significantly better overall survival than other patients (P=0.011).
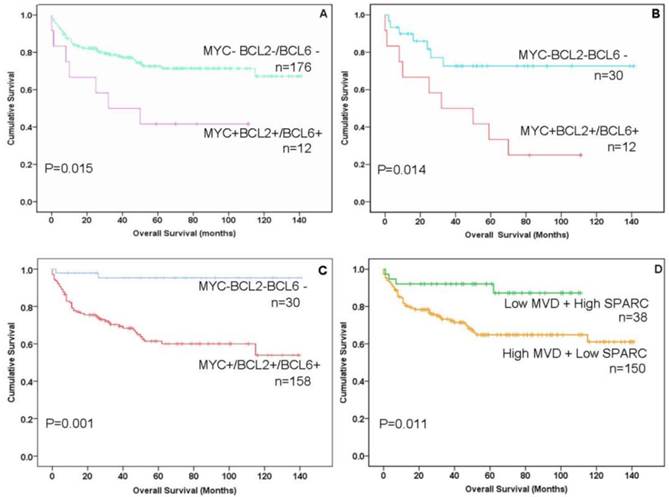
On the other hand, MVD was evaluated by CD31 expression in small vessels. High MVD was associated with poor prognosis (CD31≥10%, P<0.05) (Fig.1R 1S; Fig. 2B; Table 2). SPARC was expressed in stromal cells, including histocytes, macrophages and some of capillaries (Fig.1T 1U). SPARC expression was a better factor for OS (SPARC≥5%, P<0.05) (Fig. 2B; Table 2). When combined the MVD and SPARC expression, CD31 (<10%) and SPACR (SPARC≥5%) combination had a better prognostic value (P<0.05) (Fig.3D; Table 2)
Additionally, Ki-67 index was also significant in different histological types when the cut-off of 40% was used (p<0.01) (Fig. 1V 1W; Table 2). But CD10 and MUM1 expression had no detectable significant difference among different histological types of stomach B-cell lymphoma (Table 2).
Treatment response and Following-up
At the time of analysis, 137 patients were alive and 51 had died. The median follow-up for the 188 patients was 6 years, ranging from 0.1-12 years. 121 (64%) patients underwent surgery, 176 (92%) received chemotherapy, and 18 (23%) received radiotherapy. The most common type of surgical treatment procedure was subtotal gastrectomy and lymph node removal. 12 (6%) received no further treatment after gastric surgery. 104 (70%) of patients took the most common chemotherapy (CHOP). 84 (45%) of patients used chemotherapy with rituximab (R-CHOP) and administered six cycles of chemotherapy, 25% were administered three cycles, and 25% were administered four or five cycles. 18 (23%) of patients who received radiotherapy were treated with extended-field irradiation to the upper and middle parts of the abdomen with 30-45Gy and also treated with chemotherapy. A total of six patients (3.2%) required emergency operations, among them, two for perforation and three for intractable bleeding. Three of these six patients (50%) received chemotherapy beforehand at a median of 19 days after the first cycle of the therapy. All patients were treated with anti-H. Pylori therapy regardless of the infectious status.
The multivariate survival analysis (Cox regression model) of conventional clinical prognostic factors and some of biomarkers claimed that MYC and BCL-2 or BCL-6 co-expression, “MYC-BCL2-BCL6” negative group, low MVD and high SPARC expression, R-CHOP chemotherapy and IPI score were independent prognostic factors (Table 3).
Discussion
As the second most common malignancy in the stomach, gastric lymphoma is getting an upward trend in both incidence and survival owing to the progress in the diagnosis and treatment in eastern and western countries in recent years [1-3]. In the daily clinical setting, the definite diagnosis, treatment management and prognostic analysis of gastric B-cell lymphomas are still essential and complicated [1]. First of all, the diagnosis of gastric B-cell lymphomas should differentiate among the reactive inflammatory conditions, MALT lymphoma and DLBCL [1-3]. Sometimes, coexist of MALT lymphoma and high grade DLBCL could make the diagnosis difficult and confusing. Gastric DLBCL may be also arise de novo without an accompanying low grade component. The distinction between DLBCL transformed from MALT lymphoma and DLBCL arising de novo is impossible [4,5]. As a practical point, the recognition of the high grade component and the distinction between a low and high grade lymphoma in the stomach could be a diagnostic challenge. Morphologic criteria for the distinction between low and high grade gastric B-cell lymphomas are not well established and even more difficult when small biopsies are used [1, 2, 4]. In order to determine the optimal management strategy for gastric lymphomas, appropriate clinical staging is also full of challenge. Actually, it is controversial as to which classification is the best system for staging of gastric lymphomas [1-3, 6-9]. Therefore, a new composite prognostic index including clinical issues, pathological morphology, and molecular biology biomarkers is imperative for clinical stratified management of gastric B-cell lymphoma.
Multivariate survival analysis (Cox regression model) of conventional clinical prognostic factors and serial biomarker expression in 188 cases of gastric B-cell lymphomas.
| Variables | Hazard | 95 % Confidence | P value |
|---|---|---|---|
| ratio | intervals | ||
| Age | 1.419 | (0.741,2.716) | 0.291 |
| Gender | 1.004 | (0.542,1.858) | 0.99 |
| Stage | 1.555 | (0.561,4.314) | 0.296 |
| IPI score | 3.74 | (2.491,5.615) | <0.001 |
| ECOG score | 2.28 | (0.906,5.740) | 0.08 |
| Surgery | 1.183 | (0.605,2.313) | 0.623 |
| R-CHOP | 0.117 | (0.041,0.333) | <0.001 |
| Surgery + CT+RT | 0.907 | (0.565,1.455) | 0.686 |
| MYC+BCL+/BCL6 + | 2.29 | (1.470,3.567) | <0.001 |
| MYC- BCL2- BCL6 - | 5.12 | (1.209,21.687) | 0.027 |
| CD31+SPARC | 0.219 | (0.063,0.763) | 0.017 |
| Ki-67 | 1.725 | (0.593,5.016) | 0.317 |
Studies in recent years have showed that molecular prognostic markers predict the prognosis for each patient's particular disease better than the classic markers [22, 23]. Over the past 5 years, several groups have correlated the presence of c-Myc translocation detected by FISH with poor outcome in DLBCL [11-13, 21]. In a recently published comprehensive analysis of the Mitelman database, 62%of “double-hit” lymphomas involve bcl-2; 18% involved bcl-6. There remaining cases were “triple-hit” lymphomas involving all 3 abnormalities [21, 22]. Although the prognoses of both “double-hit” lymphoma, defined either by FISH or “double-protein” lymphoma defined by immunohistochemistry, are clearly poor with R-CHOP, there are limited data evaluating alternative therapy, and no published prospective data focused on patients with gastric lymphomas [3, 22-25].
In the present study, we focused on evaluating serial biological molecular markers including those related to “double-hit” and “double-protein” using FISH technology and immunohistochemistry in FFPE tumor tissues of gastric B-cell lymphomas. Results of this study showed that there were a few cases with c-Myc (5.8%) or bcl-6 (7.4%) rearrangement in gastric B-cell lymphomas, while there were just one case detected with bcl-2 rearrangement and one case with t (14;18) IgH/bcl2 of DLBCL in 188 cases of gastric B-cell lymphomas. The c-Myc gene rearrangement was seen in two cases and copy number increase in one case under MALT-BCL, but MYC expression was seen only in one case. In that case, c-MYC gene rearrangement/copy number increase did not increase MYC expression, neither in two other MALT-BCL cases. These results were just same as the research of “double-hit” and “double-protein” lymphomas did not have consensus [10-12,21, 22].
However, many cases showed amplification or copy gains with c-Myc (10.1%), bcl-2 (17.0%) and bcl-6 (0.9%) respectively. Most of them were diagnosed as DLBCL. Although the positive cases were too few, it is very interesting that 9 (4.8%) cases with double changes (either rearrangement or amplification/copy gains) of c-Myc and / or bcl-2 and /or bcl-6 died of DLBCLs early during the following-up period. There were only 2 (1.1%) cases of patients with triple changes (rearrangement or amplification / copy gains) of these three genes died of DLBCL in less than one year after the initial diagnosis regardless of their treatment. As to the expression of MYC, BCL2 and BCL6, BCL2 expression was statistically significant, while neither MYC nor BCL6 expression had significant difference among different histological types of gastric B-cell lymphomas. But when comparing the expression of MYC, BCL2 and BCL6 with clinical data and following-up data, we can conclude that the co-expression of MYC and BCL-2 or BCL-6 were significantly associated with poor outcome in clinical setting and OS. As we know, MYC (c-Myc) expression is related to more aggressive lymphomas, in this retrospective study, most patients with “double-protein” of MYC and BCL2 or BCL6 co-expression were associated with poor prognosis and with the diagnosis as DLBCL or DLBCL with MALT features. But patients with “double-protein” lymphoma in stomach treated by R-CHOP did not shows statistically significant of poor prognosis and R-CHOP chemotherapy was a better treatment modality than the regime without rituximab in our cases. This result is different from previous reports that “double-protein” lymphoma was clearly poor with R-CHOP [21-23]. The discrepancy could be due to the low incidence of “double-protein” in stomach B-cell lymphoma. Importantly, the “MYC-BCL2-BCL6” negative group has been associated with a 100% 5-year survival rate compared with “double-protein” lymphoma in any pathological types of gastric B-cell lymphomas. Patients with this phenotype could be considered eligible for mild treatment instead of more invasive methods regardless of pathological types, even when the tumor is not at stage I.
On the other hand, it is well known that the tumor neoangiogensis and stromal reactivity also play an important role in tumor development and progression. This study showed low MVD detected using CD31(less than10%) in tumor tissue and high SPARC rate in stromal reactive cells, especially histocytes and macrophages (more than 5%) have a better prognosis of gastric B-cell lymphomas. SPARC is expressed in specific populations of macrophages associated with tissue injury and in a variety of tumor types, usually mesenchymal tumors [17-19, 24]. The prognostic implications of SPARC expression depend on the tumor type [17,18]. The mechanism by which stromal cell expression of SPARC affects tumor progression and patient survival is uncertain. Part of this uncertainty is due to the fact that, depending on the type of tumor and whether SPARC is present in tumor cells or non-tumor cells. SPARC expression can promote or suppress tumor growth [18, 24-27]. In lymphomas or leukemia in which the tumor cells express high levels of SPARC, such as mantle cell lymphoma, high levels of SPARC are associated with increased tumor growth [25-27]. Our findings showed that SPARC positive stromal cells (more than 5%) in the microenvironment of gastric B-cell lymphomas are associated with increased patient survival, presumably owing to tumor suppression compared with patients with those with less SPARC positive stromal cells (less than 5%), similar to the study of SPARC in DLBCL [14,26]. On the other hand, the present study showed MVD in gastric B-cell lymphoma tissue increased as tumor aggressiveness. The degree of MVD was associated with the aggressiveness and prognosis of B cell lymphomas [14,15, 26-28]. Xie et al. reported that the VEGF expression and MVD in tumor tissues were higher in gastric lymphoma than those in normal tissues [29]. Combining the multivariable analysis, our biologic prognostic model could be used in conjunction of the MVD with SPARC, and a serial biomarkers including MYC, BCL2 and BCL6 for stratifying management of patients with gastric B-cell lymphomas in clinical risk-adapted therapies in the future [26,29].
Also, there are some other factors should be considered. Ki-67 index was also significantly different in different histological types when the cut-off level of 40% was used in this study. This was a helpful marker to make differentiate diagnosis of MALT lymphoma or DLBCL in gastric B-cell lymphomas [1, 23]. But significant difference of CD10 and MUM1 expression were not obtained among different histological types of gastric B-cell lymphomas. The gastrectomy in the management of gastric lymphoma has been nowadays also reserved for those patients with disease- or treatment-related complications due to potentially increasing the risk of morbidity and mortality [8,9,30]. In this study, surgery was a mainly treatment to patients at stage II or higher. The incidence of chemotherapy/radiotherapy-related gastric perforation or hemorrhage in this study was lower than those in other reports [8,9,30]. Although there are recent debates on the necessity of the invasive modality, compared with conservative treatment to gastric lymphomas, we suggest that surgery is still the best way to prevent chemotherapy/ radiotherapy related complication and tumor recurrence [30]. For patients infected with H. Pylori, antibiotic treatment of H. Pylori is effective and seems to be the first-line therapy, especially to the localized low grade gastric lymphoma [4, 5]. But in the present study, the diseases with antibiotic treatment of H. pylori recurred quite often. Indeed, H. pylori status was difficult determined in recurrence and high grade cases in clinical setting. So we suggested that radical treatment for H. pylori is necessary to all patients of gastric lymphomas [1, 4, 5, 31]. Whatever the treatment modality is, careful patient follow-up via ultrasonography and endoscopic biopsies is paramount in preventing gastric lymphomas relapses and ensuring maximizing disease-free survival for the patients' management [1, 23, 29, 32].
In summary, the “double-hit” is rare among primary gastric B-cell lymphomas, while patients with multiple gene amplification and/or copy gains of c-Myc, bcl2 and bcl6, and “double-protein” in gastric B-cell lymphomas were relatively common, which had a poor clinical outcome. In addition, the patients with “MYC-BCL2-BCL6” negative, low MVD in tumor tissue with high expression of SPARC in stromal cells had a better prognostic model regardless of clinical stage and pathological types. These results could be a potential useful molecular pathology prognostic model for clinical stratified management and precision medicine of gastric B-cell lymphomas.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Project No. 81372544).
Author Contributions
Conceived and designed the experiments: MX H, JM Y, JM Z. Performed the experiments: KT C, MX H. Analyzed the data: MX H, KT C, SM Z, SH L, XX H, L G, J C, XM S, WP ZH, JM W. Contributed reagents/materials/analysis tools: MX H KT C. Wrote the paper: MX H, SM Z, JM Y, JM Z.
Competing Interests
The authors declare that they have no competing interests.
References
1. Swerdlow SH, Campo E, Harris NL. et al. The World Health OrganizationClassification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: WHO. 2008
2. Terada T. Histopathological study using computer database of 10 000 consecutive gastric specimens: (2) malignant lesions. Gastroenterol Rep (Oxf). 2015:1-5
3. Chen Y, Chen Y, Chen S. et al. Primary Gastrointestinal Lymphoma: A Retrospective Multicenter Clinical Study of 415 Cases in Chinese Province of Guangdong and a Systematic Review Containing 5075 Chinese Patients. Medicine (Baltimore). 2015;94:e2119
4. Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2015 Nov 24
5. Foster LH, Portell CA. The role of infectious agents, antibiotics, and antiviral therapy in the treatment of extranodal marginal zone lymphoma and other low-grade lymphomas. Curr Treat Options Oncol. 2015;16:28
6. Koch P, Probst A, Berdel WE. et al. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol. 2005;23:7050-7059
7. Fischbach W, Schramm S, Goebeler E. Outcome and quality of life favour a conservative treatment of patients with primary gastric lymphoma. Z Gastroenterol. 2011;49:430-435
8. MacQueen IT, Shannon EM, Dawes AJ. et al. The Role of Surgery in the Clinical Management of Primary Gastrointestinal Non-Hodgkin's Lymphoma. Am Surg. 2015;81:988-994
9. Paulus EM, Fleming MD, Hendrix AA. et al. The evolving role of surgery for gastric lymphoma: from curative resection to surgical management of complications. Am Surg. 2014;80:E322-324
10. Pon JR, Marra MA. Clinical impact of molecular features in diffuse large B-cell lymphoma and follicular lymphoma. Blood. 2016;127:181-186
11. Horn H, Ziepert M, Becher C. et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253-2263
12. Swerdlow SH. Diagnosis of 'double hit' diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program. 2014;2014:90-99
13. O'Malley DP, Auerbach A, Weiss LM. Practical Applications in Immunohistochemistry: Evaluation of Diffuse Large B-Cell Lymphoma and Related Large B-Cell Lymphomas. Arch Pathol Lab Med. 2015;139:1094-1107
14. Burger JA, Ghia P, Rosenwald A. et al. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367-3375
15. Alizadeh AA, Gentles AJ, Alencar AJ. et al. Prediction of survival in diffuse large B-cell lymphoma based on the expression of 2 genes reflecting tumor and microenvironment. Blood. 2011;118:1350-1358
16. Sagaert X, Van Cutsem E, De Hertogh G. et al. Gastric MALT lymphoma: a model of chronic inflammation-induced tumor development. Nat Rev Gastroenterol Hepatol. 2010;7:336-346
17. Lenz G, Wright G, Dave SS. et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313-2323
18. Meyer PN, Fu K, Greiner T. et al. The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol. 2011;135:54-61
19. Cardesa-Salzmann TM, Colomo L, Gutierrez G. et al. High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica. 2011;96:996-1001
20. Zhang S, Wei M, Liang Q. et al. The t (14;18)(q32;q21)/IGH-MALT1 translocation in gastrointestinal extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). Histopathology. 2014;64(6):791-798
21. Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol. 2015;16:e555-567
22. Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120:3884-3895
23. Schmidt MT, Huang Q, Alkan S. Aggressive B-cell lymphomas: a review and practical approach for the practicing pathologist. Adv Anat Pathol. 2015;22:168-180
24. Wang XJ, Medeiros LJ, Lin P. et al. MYC cytogenetic status correlates with expression and has prognostic significance in patients with MYC/BCL2 protein double-positive diffuse large B-cell lymphoma. Am J Surg Pathol. 2015;39:1250-1258
25. Friedberg JW. Using the pathology report in initial treatment decisions for diffuse large B-cell lymphoma: time for a precision medicine approach.Hematology Am Soc Hematol Educ Program. 2015. 2015:618-624
26. Porter PL, Sage EH, Lane TF. et al. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791-800
27. Brandt S, Montagna C, Georgis A. et al. The combined expression of the stromal markers fibronectin and SPARC improves the prediction of survival in diffuse large B-cell lymphoma. Exp Hematol Oncol. 2013;2:27
28. Sangaletti S, Tripodo C, Portararo P. et al. Stromal niche communalities underscore the contribution of the matricellular protein SPARC to B-cell development and lymphoid malignancies. Onco immunology. 2014;3:e28989
29. Xie L, Shen LD, Qing C. et al. Correlational study of vascular endothelial growth factor expression and microvesseldensity in primary malignant gastric lymphoma. Med Oncol. 2012;29:1711-1715
30. Mehmet K, Sener C, Uyeturk U. et al. Treatment modalities in primary gastric lymphoma: the effect of rituximab and surgical treatment. A study by the Anatolian Society of Medical Oncology. Contemp Oncol (Pozn). 2014;18:273-278
31. Hu S, Xu-Monette ZY, Tzankov A. et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021-4031
32. Zhou Z, Sehn LH, Rademaker AW. et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837-842
Author contact
![]() Corresponding authors: Jianmin Yang, Department of Hematology, Changhai Hospital, The Second Military Medical University, Shanghai, 200433, China (yangjianminorg.cn); Jianming Zheng, Department of Pathology, Changhai Hospital, The Second Military Medical University, Shanghai, 200433, China (jmzheng1962com).
Corresponding authors: Jianmin Yang, Department of Hematology, Changhai Hospital, The Second Military Medical University, Shanghai, 200433, China (yangjianminorg.cn); Jianming Zheng, Department of Pathology, Changhai Hospital, The Second Military Medical University, Shanghai, 200433, China (jmzheng1962com).

 Global reach, higher impact
Global reach, higher impact