3.2
Impact Factor
ISSN: 1837-9664
J Cancer 2015; 6(10):990-995. doi:10.7150/jca.11650 This issue Cite
Research Paper
Increased Expression of Eps15 Homology Domain 1 is Associated with Poor Prognosis in Resected Small Cell Lung Cancer
1. The Fourth Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China
2. The Department of Endoscopy, Harbin Medical University Cancer Hospital, Harbin, China
† These authors contribute equally to this work.
Received 2015-1-20; Accepted 2015-6-13; Published 2015-8-20
Abstract
One of the great challenges of small cell lung cancer (SCLC) treatment is identifying patients at high risk for recurrence after surgical resection and chemotherapy. We examined Eps15 homology domain 1 (EHD1) protein expression in paraffin sections of 85 resected SCLC tissues, metastatic lymph nodes and normal bronchial epithelial tissues using immunohistochemistry to study the correlation between EHD1 expression and patient clinicopathological features. Within these variables, disease free survival (DFS) analyzed by the log-rank test was constructed using the multivariate Cox proportional hazards regression model and Kaplan-Meier analysis. Immunohistochemistry results showed that EHD1 protein was significantly increased in SCLC tissues compared with normal tissues (P < 0.001). Moreover, EHD1 expression was positively correlated with tumor size (P = 0.019). Multivariate Cox proportional hazards model analysis showed that EHD1 expression (P = 0.047; HR, 1.869; 95% CI, 1.008-3.466) and American Joint Committee on Cancer (AJCC) status (P < 0.001; HR, 1.412; 95% CI, 1.165-1.711) were independent prognostic indicators of DFS. In conclusion, these data demonstrated a remarkable correlation between the cytoplasmic expression of EHD1 protein and adverse prognosis in patients receiving early-stage cisplatin treatment for resected SCLC.
Keywords: Eps15 homology domain 1, Disease-free survival, Small cell lung cancer, Survival, Immunohistochemistry
Introduction
Lung cancer is the most common neoplasm worldwide, with approximately 1.6 million new cases and 1.4 million deaths occurring each year [1, 2]. Small cell lung cancer (SCLC), which originates from neuroendocrine-cell precursors, accounts for up to 15% of all newly diagnosed lung cancers [3, 4]. SCLC is also the most aggressive subtype of lung cancer, with a high risk for early locoregional and distant metastases and a post-treatment relative 5-year survival rate of only 6.4% [5]. To date, platinum-based chemotherapy has been the principal treatment for SCLC patients at initial diagnosis [6-8]. However, due to primary or secondary resistance to chemotherapy, most SCLC patients rarely survive beyond 2 years from the time of diagnosis [9-11].
Mammalian Eps15 homology domain 1 (EHD1) is found on the chromosomal band 11q13, which has been found to be amplified or rearranged in plenty of carcinomas including lung, breast, head and neck and associated with poor prognosis[12-17]. EHD1 plays an important role in the regulation of various cellular events [18], including the recycling of proteins to the plasma membrane [19]. Notably, one of the most important proteins regulated in this manner are the β1 integrins [20], which bind to the extracellular matrix (ECM) and stimulate signaling pathways that influence proliferation, apoptosis, cell spreading, migration, invasion and metastasis [21-23]. The results of recent studies suggest that EHD1's role in vesicle trafficking may also be related to cancer invasion and metastasis [24, 25].
A positive correlation between the expression of EHD1 and poor survival has been observed in non-small cell lung cancer (NSCLC) [26, 27]. The aim of this study was to investigate the ability of EHD1 to serve as a prognostic marker of SCLC. To assess this prognostic capacity, the expression of EHD1 protein in tumor, metastatic lymph node and normal bronchial epithelial tissues from 85 resected SCLC patients was measured using immunohistochemistry (IHC) and correlated with certain clinicopathological features of patients.
Materials and methods
Tissue samples and patients
To assess the prognostic capacity of EHD1 for SCLC, formalin-fixed, paraffin embedded (FFPE) SCLC tumor, lymph node metastases and normal bronchial epithelial tissues were collected from 85 SCLC patients who underwent surgery between January 2008 and November 2011. Inclusion criteria were provided to be SCLC by pathology reports and included from stage I to stage IIIA. No patient received chemotherapy or radiotherapy prior to surgery. Four to six cycles of platinum-based adjuvant chemotherapy were administered to all patients. All cases representing a spectrum of SCLC were retrieved from Harbin Medical University Cancer Hospital. Exclusion criteria were stage IV of disease and history of other cancers. For each patient, each tissue specimen type was resected during the same surgical procedure. Primary cancers were evaluated in accordance with the 7th edition of the American Joint Committee on Cancer (AJCC) staging system (TNM). All patients were followed until death or the study closing date (January 21, 2014). The median follow-up time for survivors was 48.70 months (range 3.00-65.70 months). The study was approved by the Ethical Committee of Harbin Medical University in Harbin, China. Informed consent was obtained from all patients. All investigators involved in the study, apart from the study statistician, were blinded to patient outcome throughout all laboratory analyses.
Immunohistochemistry
To detect the expression of EHD1 in FFPE sections, IHC was performed as described. The tissue sections were first dried at 70°C for 3 h. After deparaffinization and hydration, sections were washed in phosphate-buffered saline (PBS; 3 × 3 min). Endogenous peroxidase was quenched with 3% H2O2 for 15 min. After washing in distilled water, sections were washed in PBS (3 × 5 min). Antigen retrieval was performed in citrate buffer (pH 6.0). Each section was then treated with 300-500 ml EHD1 rabbit polyclonal antibody (Abcam, Cambridge, UK, ab75886, diluted at 1:200) solution overnight at 4°C. The sections were incubated with peroxidase-conjugated streptavidin for 30 min, and the reaction products were visualized with diaminobenzidine as a chromogen and counterstained with commercial hematoxylin. The percentage of positive cells was determined by counting 500 cells in five random selected fields per section. IHC staining was scored based on intensity, as follows: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown) and 3 (strong staining = brown). The percentage (0-100%) of the extent of reactivity was scored as follows: 0 (no positive tumor cells), 1 (fewer than 10% positive tumor cells), 2 (10-50% positive tumor cells) and 3 (greater than 50% positive tumor cells). Next, the cytoplasmic expression score was obtained by multiplying the intensity and reactivity rate values. Scores of < 4 were classified as low expression, and the remainders were classified as high expression. Two blinded independent observers interpreted all slides.
Statistical analysis
All analyses were performed using statistical software (SPSS 13.0 for Windows; SPSS, Inc., Chicago, IL, USA). Differences were considered statistically significant when P values were < 0.05. Correlation of EHD1 expression levels between tissue types and correlation of EHD1 expression levels in tissues with clinicopathological features of patients were assessed using chi-square tests. Disease-free survival (DFS) were calculated from the date of surgery resection to the date of last follow-up or relapse. Cumulative DFS curves were plotted by the Kaplan-Meier method, and the relationship between each of the variables and survival was assessed by log-rank test in univariate analysis. The covariates with P ≤ 0.15 in univariate analyses were adopted into multivariate analyses. The parameters were then tested by the multivariate Cox proportional hazards model, which was performed to identify independent variables for predicting survival. Risk ratios and their 95% confidence intervals (CIs) were recorded for each factor.
Results
EHD1 protein expression in tumor, lymph node metastases and normal bronchial epithelial tissues
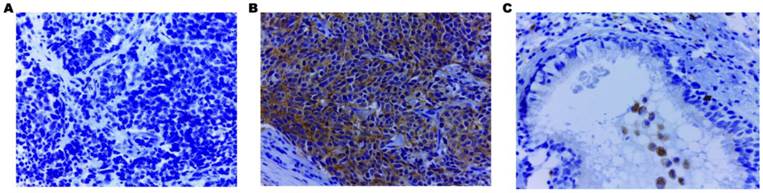
Using IHC, EHD1 protein expression was examined in tumor, lymph node metastases and normal bronchial epithelial tissues of 85 SCLC patients. The frequency of positive staining for EHD1 was 58.82%(50/85) in the SCLC samples, which was significantly higher (P < 0.001) than that in the normal tissues(21.18%) (Table 1). The cytoplasmic staining patterns observed for EHD1 were consistent with data from our previous studies (Fig. 1) [26, 27].
Association of EHD1 protein expression and clinicopathological features
Correlation of EHD1 expression levels with a range of clinicopathological features of patients, including age, gender, smoking history, adjuvant radiotherapy history, tumor histology, tumor size, presence of lymph node metastasis (LNM) and AJCC stage in SCLC patients (Table 2), was assessed. High expression of EHD1 in the cytoplasm was only positively correlated with tumor size (P = 0.019). No such significant correlations between EHD1 and other clinicopathological features were found in this study.
Univariate and multivariate Cox regression analysis of potential prognostic indicators of DFS in SCLC patients
In all patients, AJCC stage and EHD1 overexpression were significantly associated with DFS based on univariate Cox regression models. Other features showed no major prognostic value. Multivariate Cox proportional hazards model analysis of the same set of patients showed that AJCC stage (P < 0.001; HR, 1.412; 95% CI, 1.165-1.711) and EHD1 (P = 0.047; HR, 1.869; 95% CI, 1.008-3.466) were independent prognostic indicators of DFS in SCLC patients (Table 3).
Expression of EHD1 in small cell lung cancer, lymph node metastases and normal bronchial epithelial tissues
| Tissue | Tissue | |||||
|---|---|---|---|---|---|---|
| Small cell lung cancera | Normalb | P | Small cell lung cancera | Lymph nodec | P | |
| <0.001 | 0.539 | |||||
| Low | 35 (41.18%) | 67 (78.82%) | 35 (41.18%) | 17 (47.22%) | ||
| High | 50 (58.82%) | 18 (21.18%) | 50 (58.82%) | 19 (52.78%) | ||
note: [0 (negative) ≤ score ≤ 1+] and [2+ ≤ score ≤ 3+] represent low negative and strong positive staining of EHD1, respectively. All the cut off points contributed to acquiring the optimum balance ratio between negative and positive. ª. Lung primary tissues; b. Normal bronchial epithelial tissues; c. Lymph node metastases.
The correlation between clinicopathological features and the expression of EHD1
| EHD1 (n=85)ª | |||
|---|---|---|---|
| Variables | Low | Strong | P |
| Age | 0.364 | ||
| ≤60 | 27(31.76%) | 39(45.88%) | |
| >60 | 10(11.76%) | 9(10.59%) | |
| Gender | 0.521 | ||
| male | 19(22.35%) | 28(32.94%) | |
| female | 18(21.18%) | 20(23.53%) | |
| Smoking history | 0.546 | ||
| never | 1(1.18%) | 4(4.71%) | |
| ever | 20(23.53%) | 25(29.41%) | |
| unknown | 16(18.82%) | 19(22.35%) | |
| Adjuvant radiotherapy | 0.828 | ||
| Yes | 10(11.76%) | 14(16.47%) | |
| No | 27(31.76%) | 34(40.00%) | |
| Tumor histology | 0.462 | ||
| pure | 35(41.18%) | 43(50.59%) | |
| combined | 2(2.35%) | 5(5.88%) | |
| Tumor Size | 0.019* | ||
| ≤3 | 14(16.47%) | 25(29.41%) | |
| >3 | 24(28.24%) | 22(25.88%) | |
| Nodal | 0.290 | ||
| negative | 22(25.88%) | 23(27.06%) | |
| positive | 15(17.65%) | 25(29.41%) | |
| AJCC Stage | 0.781 | ||
| I | 17(20.00%) | 20(23.53%) | |
| II | 11(12.94%) | 13(15.29%) | |
| III | 9(10.59%) | 15(17.65%) | |
note: [0 (negative) ≤ score ≤ 1+] and [2+ ≤ score ≤ 3+] represent low negative and strong positive staining of EHD1, respectively. All the cut off points contributed to acquiring the optimum balance ratio between negative and positive. * P < 0.05. ª. Three EHD1 samples were excluded because of damage to or cytolysis of the paraffin block.
EHD1 expression in primary tumor tissues as an independent prognostic factor for DFS in resected small cell lung cancer patients
| Variables | Univariablea | Multivariableb | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.004 (0.966-1.043) | 0.840 | 0.155 | |
| Gender | 1.364 (0.771-2.411) | 0.286 | 0.250 | |
| Smoking history | 0.766 (0.468-1.287) | 0.326 | 0.854 | |
| Adjuvant radiotherapy | 1.252 (0.933-1.679) | 0.134 | 0.066 | |
| Tumor histology | 0.695 (0.259-1.865) | 0.470 | 0.331 | |
| AJCC stage | 1.439 (1.182-1.752) | <0.001 | 1.412 (1.165-1.711) | 0.001 |
| EHD1 expression | 1.958 (1.060- 3.615) | 0.032 | 1.869 (1.008-3.466) | 0.047 |
note: CI, confidence interval; HR, hazard ratio; DFS, disease free survival. a Variables were adopted for their prognostic significance (P < 0.05) in univariate analysis using forward, stepwise selection (forward likelihood ratio). b A Cox proportional hazards regression model was used for multivariate analysis.
Kaplan-Meier survival curve analysis of DFS in SCLC patients and correlation of EHD1 expression
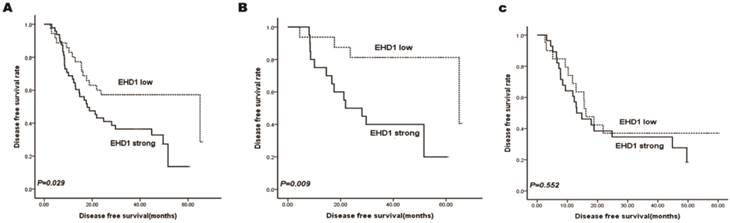
Kaplan-Meier survival curves stratified for EHD1 expression are shown in Fig. 2a. EHD1 expression showed significant effects on DFS (P = 0.029), specifically that high EHD1 expression was associated with poor DFS. Additionally, two subgroups were classified according to AJCC stage status (Fig. 2b, 2c). Accordingly, the prognosis for patients with low expression of EHD1 was significantly better (P = 0.009) than for patients with high expression of EHD1 in stage I SCLC, whereas EHD1 expression was not a significant predictor of DFS in stage II and IIIA SCLC (P = 0.552).
Immunohistochemical staining of EHD1 in FFPE tissue samples (×400). (A) Cytoplasmic EHD1-negative specimen (SCLC). (B) Cytoplasmic EHD1 high-expression specimen (SCLC). (C) Cytoplasmic EHD1 low-expression specimen (normal bronchial epithelial tissue).
Kaplan-Meier analysis for disease free survival (DFS) based on EHD1 expression status in SCLC patients. (A-C)Kaplan-Meier analysis for DFS based on EHD1 expression status in (A) all patients (B) stage I SCLC patients (C) stage II and IIIA SCLC patients.
Discussion
The results of this study revealed that the expression of EHD1 predicted DFS in a cohort of 85 SCLC patients who received a surgical resection and platinum-based adjuvant chemotherapy, which is a similar result to that found in our previous study reporting the prognostic value of EHD1 expression in NSCLC [26, 27]. Platinum-based treatment is a crucial part of the standard regimen for SCLC and NSCLC patients. Recent research showed that high mRNA levels of EHD1 were associated with cisplatin resistance in HeLa cells [28]. High levels of EHD1 expression are also associated with poor response to treatment in cutaneous T cell lymphoma [29]. These results suggested that EHD1 might mediate cisplatin resistance through regulating endocytosis.
Our study reveals that EHD1 overexpression was markedly correlated with tumor size. It suggests that the EHD1 expression level might provide information that correlates with the ability of tumor cell proliferation. Other studies observed the involvement of misregulated and mutated EHD1 in cancer progression [19, 20], invasion and metastasis [25]. EHD1 is primarily involved in tubular recycling endosome (TRE) membrane vesiculation [30], acting as a gatekeeper to promote the recycling of a variety of receptors from the endocytic recycling compartment (ERC) to the plasma membrane [19, 31-36]. For example, EHD1 protein is involved in endocytosis and trafficking of various membrane proteins including major histocompatibility complex (MHC) class proteins [33], insulin-like growth factor 1 (IGF1) [37, 38] and secretion of glucose transporter 4 (GLTU-4) [36, 39]. Therefore, it is possible that the loss of EHD1 activity could give rise to tumor development through the aberrant regulation of MHC class molecules, which participate in antigen presentation and destruction of abnormal cells [36]. Furthermore, some studies reported that IGF1 is associated with diverse types of cancer, such as pancreatic cancer [40], leukemia [41], hepatocarcinoma (HCC) [42] and breast cancer [43], and Zheng X et al. reported that GLUT4 protein is a common disruptor of carbohydrate metabolism [44], which is linked to elevated cancer risk. Additionally, EHD1 and its interaction partner molecule interacting with CasL-like protein 1 (MICAL-L1) are required for the activation and transport of Src, which plays a primary role in regulating cell adhesion and migration [45], and the misregulation of Src kinase activity in cancer is related to metastasis and poor survival [46]. As previously mentioned, β1 integrins stimulate signaling pathways affecting cell motility and migration as well as tumor cell invasiveness [21], adhesion and spreading [22, 23]. EHD1 plays a significant role in regulating β1 integrin transport and is further involved in integrin-mediated downstream functions [20]. One study indicated that aberrant regulation of EHD1 could possibly lead to tumor development, as was shown in metastatic colon cancer, non-Hodgkin lymphoma (NHL) and partially in rhabdomyosarcoma (RMS) [24].
Summarily, EHD1 expression may affect the sensitivity of tumor cells to cisplatin-containing treatment and recurrence or metastasis through the regulation of the endocytic recycling process.
Notwithstanding, EHD1 expression status did not correlate with overall survival (OS). SCLC is an aggressive malignancy with extremely high recurrence and metastasis, though the mechanism by which it progresses is not yet clear. Recent experimental observations identified many signaling molecules, such as CEA, MGr1-Ag and receptor tyrosine kinases (RTKs), involved in its regulation [47-49]. We purport that EHD1 is an effective prognostic marker for SCLC patients, particularly for those in the early stages of disease; however, as SCLC progresses and other molecular mechanisms take over, the effect of EHD1 is reduced. Therefore, further research is required to identify the molecular mechanisms underlying EHD1 protein overexpression and its role in SCLC. A larger sample size is also needed to verify the results of this study. In conclusion, this study presents evidence that EHD1 may predict the prognosis of early stage SCLC patients receiving cisplatin treatment.
Abbreviations
EHD1: Eps15 Homology Domain 1, IHC: immunohistochemistry, FFPE: formalin-fixed paraffin-embedded, SCLC: small cell lung cancer, DFS: disease-free survival.
Acknowledgements
We would like to thank to Professor Jin Xiaoming and Dr. Tong Dandan for providing pathologic evaluation.
This work was supported by the National Natural Science Foundation and by Natural Science Foundation of Heilongjiang Province, China (grant number LC2012C08) and by the Science Foundation of China (grant number 30772540, 81172214, 81301991).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69-90 doi:10.3322/caac.20107
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11-30 doi:10.3322/caac.21166
3. Oberg K, Hellman P, Kwekkeboom D, Jelic S, Group EGW. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 5):v220-2 doi:10.1093/annonc/mdq191
4. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A. et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:4539-44 doi:10.1200/JCO.2005.04.4859
5. Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N. et al. SEER Cancer Statistics Review, 1975-2006. National Cancer Institute.Bethesda: National Cancer Institute. 2009
6. 1088366Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ. et al. Small cell lung cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11:78-98
7. Pujol JL, Carestia L, Daures JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. British journal of cancer. 2000;83:8-15 doi:10.1054/bjoc.2000.1164
8. Johnson BE, Janne PA. Basic treatment considerations using chemotherapy for patients with small cell lung cancer. Hematology/oncology clinics of North America. 2004;18:309-22 doi:10.1016/j.hoc.2003.12.008
9. Murray N, Turrisi AT 3rd. A review of first-line treatment for small-cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2006;1:270-8
10. Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology. 2008;22:1486-92
11. Davies AM, Lara PN, Lau DH, Gandara DR. Treatment of extensive small cell lung cancer. Hematology/oncology clinics of North America. 2004;18:373-85 doi:10.1016/j.hoc.2003.12.012
12. Wangpaichitr M, Sullivan EJ, Theodoropoulos G, Wu C, You M, Feun LG. et al. The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells. Molecular cancer therapeutics. 2012;11:604-15 doi:10.1158/1535-7163.MCT-11-0599
13. Wangpaichitr M, Wu C, You M, Maher JC, Dinh V, Feun LG. et al. N',N'-Dimethyl-N',N'-bis(phenylcarbonothioyl) Propanedihydrazide (Elesclomol) Selectively Kills Cisplatin Resistant Lung Cancer Cells through Reactive Oxygen Species (ROS). Cancers. 2009;1:23-38 doi:10.3390/cancers1010023
14. Schwab M. Amplification of oncogenes in human cancer cells. BioEssays: news and reviews in molecular, cellular and developmental biology. 1998;20:473-9 doi:10.1002/(SICI)1521-1878(199806)20:6<473::AID-BIES5>3.0.CO;2-N
15. Dickson C, Fantl V, Gillett C, Brookes S, Bartek J, Smith R. et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer letters. 1995;90:43-50
16. Gollin SM. Chromosomal alterations in squamous cell carcinomas of the head and neck: window to the biology of disease. Head & neck. 2001;23:238-53
17. Xu J, Tyan T, Cedrone E, Savaraj N, Wang N. Detection of 11q13 amplification as the origin of a homogeneously staining region in small cell lung cancer by chromosome microdissection. Genes, chromosomes & cancer. 1996;17:172-8 doi:10.1002/(SICI)1098-2264(199611)17:3<172::AID-GCC5>3.0.CO;2-1
18. Sato M, Sato K, Liou W, Pant S, Harada A, Grant BD. Regulation of endocytic recycling by C. elegans Rab35 and its regulator RME-4, a coated-pit protein. The EMBO journal. 2008;27:1183-96 doi:10.1038/emboj.2008.54
19. Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends in cell biology. 2011;21:122-31 doi:10.1016/j.tcb.2010.10.003
20. Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. Journal of cell science. 2007;120:802-14 doi:10.1242/jcs.03383
21. Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14-21 doi:10.1111/j.1600-0854.2005.00362.x
22. Dunphy JL, Moravec R, Ly K, Lasell TK, Melancon P, Casanova JE. The Arf6 GEF GEP100/BRAG2 regulates cell adhesion by controlling endocytosis of beta1 integrins. Current biology: CB. 2006;16:315-20 doi:10.1016/j.cub.2005.12.032
23. Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Current biology: CB. 2001;11:1392-402
24. Pal NR, Aguan K, Sharma A, Amari S. Discovering biomarkers from gene expression data for predicting cancer subgroups using neural networks and relational fuzzy clustering. BMC bioinformatics. 2007;8:5. doi:10.1186/1471-2105-8-5
25. Kamens AJ, Eisert RJ, Corlin T, Baleja JD, Kritzer JA. Structured Cyclic Peptides That Bind the EH Domain of EHD1. Biochemistry. 2014;53:4758-60 doi:10.1021/bi500744q
26. Gao Y, Wang Y, Sun L, Meng Q, Cai L, Dong X. Expression of TGFbeta-1 and EHD1 correlated with survival of non-small cell Lung cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014 doi:10.1007/s13277-014-2164-x
27. Lu H, Meng Q, Wen Y, Hu J, Zhao Y, Cai L. IncreasedEHD1 in non-small cell lung cancer predicts poor survival. Thoracic Cancer. 2013;4:422-32
28. Wu ZZ, Lu HP, Chao CC. Identification and functional analysis of genes which confer resistance to cisplatin in tumor cells. Biochemical pharmacology. 2010;80:262-76 doi:10.1016/j.bcp.2010.03.029
29. Shin J, Monti S, Aires DJ, Duvic M, Golub T, Jones DA. et al. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood. 2007;110:3015-27 doi:10.1182/blood-2006-12-061507
30. Cai B, Giridharan SS, Zhang J, Saxena S, Bahl K, Schmidt JA. et al. Differential roles of C-terminal Eps15 homology domain proteins as vesiculators and tubulators of recycling endosomes. The Journal of biological chemistry. 2013;288:30172-80 doi:10.1074/jbc.M113.488627
31. Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nature cell biology. 2001;3:567-72 doi:10.1038/35078543
32. Naslavsky N, Boehm M, Backlund PS Jr, Caplan S. Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Molecular biology of the cell. 2004;15:2410-22 doi:10.1091/mbc.E03-10-0733
33. Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG. et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. The EMBO journal. 2002;21:2557-67 doi:10.1093/emboj/21.11.2557
34. Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. American journal of physiology Cell physiology. 2003;285:C1009-18 doi:10.1152/ajpcell.00140.2003
35. Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nature reviews Molecular cell biology. 2006;7:897-908 doi:10.1038/nrm2060
36. Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH? Journal of cell science. 2005;118:4093-101 doi:10.1242/jcs.02595
37. Dealy CN, Kosher RA. IGF-I and insulin in the acquisition of limb-forming ability by the embryonic lateral plate. Developmental biology. 1996;177:291-9 doi:10.1006/dbio.1996.0163
38. Lok F, Owens JA, Mundy L, Robinson JS, Owens PC. Insulin-like growth factor I promotes growth selectively in fetal sheep in late gestation. The American journal of physiology. 1996;270:R1148-55
39. Guilherme A, Soriano NA, Furcinitti PS, Czech MP. Role of EHD1 and EHBP1 in perinuclear sorting and insulin-regulated GLUT4 recycling in 3T3-L1 adipocytes. The Journal of biological chemistry. 2004;279:40062-75 doi:10.1074/jbc.M401918200
40. Burney S, Irfan K, Saif MW, Masud F. Diabetes and pancreatic cancer. JOP: Journal of the pancreas. 2014;15:319-21 doi:10.6092/1590-8577/2680
41. Benabbou N, Mirshahi P, Bordu C, Faussat AM, Tang R, Therwath A. et al. A subset of bone marrow stromal cells regulate ATP-binding cassette gene expression via insulin-like growth factor-I in a leukemia cell line. International journal of oncology. 2014 doi:10.3892/ijo.2014.2569
42. Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma non-alcoholic fatty liver disease? World journal of gastroenterology: WJG. 2014;20:9217-28 doi:10.3748/wjg.v20.i28.9217
43. Sarkissyan S, Sarkissyan M, Wu Y, Cardenas J, Koeffler HP, Vadgama JV. IGF-1 Regulates Cyr61 Induced Breast Cancer Cell Proliferation and Invasion. PloS one. 2014;9:e103534. doi:10.1371/journal.pone.0103534
44. Zheng X, Xu M, Fang Q. Role of AMPK alpha in Skeletal Muscle Glycometabolism Regulation and Adaptation in relation to Sepsis. BioMed research international. 2014;2014:390760. doi:10.1155/2014/390760
45. Fincham VJ, Frame MC. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. The EMBO journal. 1998;17:81-92 doi:10.1093/emboj/17.1.81
46. Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. The oncologist. 2009;14:667-78 doi:10.1634/theoncologist.2009-0009
47. Heidemann F, Schildt A, Schmid K, Bruns OT, Riecken K, Jung C. et al. Selectins mediate small cell lung cancer systemic metastasis. PloS one. 2014;9:e92327. doi:10.1371/journal.pone.0092327
48. Zhang F, Wang Y, Xu M, Dong H, Liu N, Zhou J. et al. MGr1-Ag promotes invasion and bone metastasis of small-cell lung cancer in vitro and in vivo. Oncology reports. 2013;29:2283-90 doi:10.3892/or.2013.2396
49. Jafri NF, Ma PC, Maulik G, Salgia R. Mechanisms of metastasis as related to receptor tyrosine kinases in small-cell lung cancer. Journal of environmental pathology, toxicology and oncology: official organ of the International Society for Environmental Toxicology and Cancer. 2003;22:147-65
Author contact
![]() Corresponding author: Li Cai, M.D., Ph.D., The Fourth Department of Medical Oncology, Harbin Medical University Cancer Hospital, Haping Road 150, Harbin, 150040, China. Phone: 86-451-86298283; Fax: 86-451-86298735; E-mail: cailihrbmu.edu.cn.
Corresponding author: Li Cai, M.D., Ph.D., The Fourth Department of Medical Oncology, Harbin Medical University Cancer Hospital, Haping Road 150, Harbin, 150040, China. Phone: 86-451-86298283; Fax: 86-451-86298735; E-mail: cailihrbmu.edu.cn.

 Global reach, higher impact
Global reach, higher impact